Journal of
eISSN: 2373-633X


Research Article Volume 4 Issue 1
Department of Chemistry, University of Kurdistan, Iran
Correspondence: Mohammad Zarei, Department of Chemistry, University of Kurdistan, Iran Sanandaj, Iran, Tel 66177-15177
Received: August 19, 2015 | Published: January 27, 2016
Citation: Zarei M. Effect of chemical radicals on the geometry of DNA: computational study. J Cancer Prev Curr Res. 2016;4(1):27-30. DOI: 10.15406/jcpcr.2016.04.00108
To understand the effect of chemical radicals on DNA base pairs, we investigate the mechanism for the reaction of a hydroxyl radical to the GC and AT base pairs, by the density functional theory (DFT) calculations. The effect of solvation on the mechanism is also presented by the same DFT calculations under the continuum solvation model. We find that, radical structure increasing the length of the nearest hydrogen bond of adjacent base pair, accompanied by decreasing the length of furthest hydrogen bond. According to the results, hydrogen bond lengths between AT an GC base pairs in water solvent are longer than vacuum.
Keywords: density functional theory, DNA, radicals, base pair, hydrogen bond, electronic properties
Chemical compounds can be transformed into free radical forms. The radical species are usually reactive and short-lived because of their single unpaired electron in their outer orbital. Studies suggested that free radical intermediates may play an important role in the toxicity of a large number of materials.1– 4 Oxygen-containing free radicals such as hydroxyl radical can be generated by various mechanisms in living organisms, including the oxidative microbicidal processes.5 Neutrophil species have been shown to produce genetic mutations in bacteria6 and cytogenetic damage to the chromosomes of mammals.7 There is a meaningful correlation between the development of cancers and the presence of radical species.7 It was observed that the DNA strand breaks induced by active oxygen species probably play a role in the DNA damage.8,9 Study of radical species impacts on biological system attract the attentions for decades but the effect of these species on biological systems such as DNA structure has not been investigated by theoretical methods and this is important to understand the mechanism of DNA damage introduced by radicals. This study provide a clear information about the influence of radicals on DNA structure and provide the mechanism which how chemical radicals could deform the DNA base pairs. This study would be applied in designing the efficient radical scavengers and anti-oxidant drugs i.e. drug design.
Structures of Watson-Crick, guanine-cytosine (GC) and adenine-thymine (AT) base pairs are made and optimized in vacuum and H2O continuum. The B3LYP functional can describe the potential energy surface for the reaction mechanisms of free radical with pyrimidine and purine structures.10 In the present DFT study, we added the radicals near the two different positions of the AT and GC base pairs: Position α and position β. In position α, radical structure placed at the top of each base pair and in position β at the bottom. These structures are optimized in vacuum by the unrestricted DFT method, as well as, to investigate the effect of solvation on the reaction mechanism between the base pair and the radicals, we performed the same DFT optimizations in H2O approximated by the continuum solvation model.11 Binding energy for all structures calculated according to equation 1:
(1) Where E (Base Pair-Radical), E Base Pair and E Radical are the total energies of base pair/radical complex, base pair and radical structures, respectively. The Mulliken population analysis was used for calculation of atomic charges. Unrestricted DFT Used for open shell molecules such as O, H2O+, OH, O. All of calculations were performed by the B3LYP method using the 6-311++G (d,p) basis set and B3PW91 level of computation. In all cases, we neglect the backbone of DNA and focus on the base pairs; all computations were carried with Gaussian 09 package.12
Optimized structures of DNA base pair + hydroxyl radical in vacuum and water
We first optimized the hydroxyl radical structures in vacuum and water. Electrical charge of atoms, also HOMO and LUMO energies of radical are calculated. Results suggested that, the electrical charges of radical atoms in H2O solvent have bigger absolute values than that of vacuum, which may be related to the fact that H2O molecules were increased the accumulation of electrical charges on radical atoms due to the inductive effects. H2O molecules influenced the energy levels of HOMO and LUMO of radicals compared to the vacuum phase. NA is surrounded by water molecules in living organisms and their influence on DNA structure should be considered. Therefore, We considered the effect of solvating water molecules by the continuum solvation model.11 AT and GC structures are optimized in both vacuum and H2O solvent (Figure 1). Structural properties of AT and GC base pairs such as hydrogen bond lengths and dihedral angles are presented in Table 1 and compared to experimental values,13-15 as can be seen there is a good correlation between the calculated values and experimental values obtained by X-ray crystallography, which confirms the precision and accuracy of calculations. The hydrogen bond length and dihedral angle between base pairs are very important factors to understand the influence of radicals on the structure and geometry of AT and GC base pairs. Hydrogen bond is defined as X—H…Y, where X—H is a proton donor and Y is proton acceptor. The length of hydrogen bond was defined as the distance between X to Y, which represents the fact that chemical properties of base pairs are influenced by equilibrium distance of atom’s center of mass and not based on the orientation of hydrogen atom which contributed in the formation of hydrogen bond.
|
Base pair |
Bond |
Bond length (Å) |
Experiment* |
|
|
Water |
Vacuum |
|||
|
N13(A)-O23(T) |
2.96 |
2.95 |
2.95 |
|
|
N11(A)-N25(T) |
2.92 |
2.88 |
2.93 |
|
|
C1(A)-O24(T) |
3.71 |
3.67 |
3.7 |
|
|
AT |
Dihedral angle |
179.98° |
179.88° |
178.1° |
|
N23(G)-O17(C) |
2.88 |
2.93 |
2.91 |
|
|
N9(G)-N18(C) |
2.96 |
2.95 |
2.95 |
|
|
O20(G)-N26(C) |
2.91 |
2.82 |
2.86 |
|
|
GC |
Dihedral angle |
179.83° |
179.92° |
178.2° |
Table 1 The structural properties of natural AT and GC base pairs in vacuum and H2O solvent. *X-ray crystallography13-15
Table 1 shows the obtained distances between AT and GC base pairs in H2O solvent and vacuum. According to the results, hydrogen bond lengths between AT and GC base pairs in H2O solvent are longer than vacuum, which may be due to the stronger molecular interactions between base pairs in vacuum compared to H2O solvent. Comparison of electrical charge of atoms shows the electrical charges of atoms in H2O solvent have a bigger absolute value compared to the vacuum. The reason is that, the H2O molecules interacted with atoms which have high absolute electrical charges and reduces the interaction of them with another base pair atoms, so disturb the electrostatic interactions between base pair atoms, and the strength of hydrogen bond between AT and GC atoms are reduced compared to the vacuum phase.
Structural and electronic properties of AT and GC base pairs + OH-radical in vacuum and water
The optimized structures of AT and GC base pairs in the presence of hydroxyl radical in positions α and β are shown in Figure 2 & Figure 3. We define four cases of radical-base pair interactions in vacuum and H2O solvent for different radical positions (α and β). According to the Figure 2, except the case of AT-OH/α-H2O, in other cases, hydroxyl radical choses the proper position for construction of hydrogen bonds with AT base pair. In positions α and β in H2O, hydroxyl radical is placed at the out of plane of AT and GC base pairs. But in vacuum phase it chose in plane position for interaction with base pairs. Table 2 shows the hydrogen bond length between AT and GC base pairs in presence of hydroxyl radical. For both AT and GC base pairs in α and β positions of interaction, hydroxyl radical increasing the length of the nearest hydrogen bond of adjacent base pair, which is accompanied by decreasing the length of furthest hydrogen bond. This phenomenon may be explained by the conservation of potential energy for interactions between radical and base pairs. Dihedral angle of base pairs also varied by hydroxyl radical, and largest change belongs to the AT base pair (169.97°). Also we can use of calculated electrical charges to interpret the hydrogen bond length variations. In vacuum and position α, which hydroxyl radical is adjacent to bond N13-O23 (AT), the electrical charge variation (ΔΕ) of N13 and O23 atoms is -0.018 and -0.099 and atoms gain electrons, which increase the repulsion forces between N13 and O23 atoms, subsequently increase the hydrogen bond length between N13 and O23 atoms up to 2%. On the other hand, the H15 which makes the hydrogen bond with O23 gains electrons (ΔΕ =-0/050) which leads to repulsion and weakening of the hydrogen bond between O23 and N13 atoms. At position β, the ΔΕ of C1 and O24 atoms is -0.105 and -0.034, so the potential energy of repulsion between C1 and O24 atoms was increased and hydrogen bond length between C1 and O24 atoms was increased up to 3%. Also for N13-O23 hydrogen bond, the ΔΕ of N13, H14, H15, and O23 atoms is -0.057, 0.029, 0.036 and 0.032 respectively, thus the columbic forces between atoms are increased which lead to shortening of hydrogen bond between N13-O23 atoms. In H2O solvent, the ΔΕ for AT base pair is negligible but the variations of dihedral angle suggested that, the AT base pair is influenced by hydroxyl radical in H2O solvent. According to results and electrical charges variations (ΔΕ), at position α of GC base pair, the ΔΕ of O17 and N23 atoms is 0.004 and 0.059, thus the repulsive interactions between two atoms increased. The ΔE for N26, H28, and O20 is -0.043, 0.036 and 0.044 respectively that increase the electrostatic interactions between atoms and reduces the length of hydrogen bond. The electronic properties of hydroxyl radical interaction with AT and GC base pairs in vacuum and H2O solvent shows that, in AT base pair the energy level of HOMO and LUMO have more negative values (HOMO (eV) = -6.421and LUMO (eV) = -1.621) compared to the natural AT (HOMO = -6.177 and LUMO= -1.442) and GC base pairs. Hydroxyl radical increases the partial conductance of AT base pairs in the vacuum; also there are similar results for GC base pairs. The calculated binding energies according to equation 1 proposes the interaction between hydroxyl radical and AT and GC base pairs is physical adsorption (Eb AT-OH (eV) =5.44, 5.17, 5.44, 5.17 and Eb GC-OH (eV) = 11.97, 5.17, 5.44, 4.63).
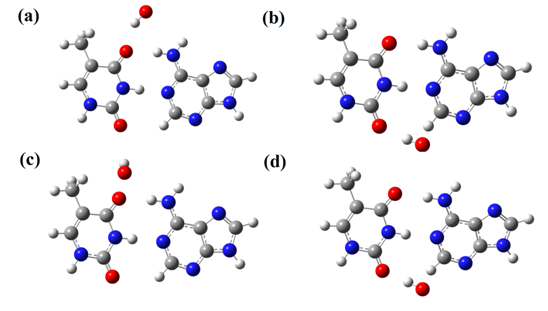
Figure 2 Optimized structures of AT base pair + OH-radical in (A) α-vacuum; (B) β-vacuum; (C) α-H2O; (d) β-H2O.

Figure 3 Optimized structures of GC base pair + OH-radical in (A) α-vacuum; (B) β-vacuum; (C) α-H2O; (d) β-H2O.
|
Pair |
bond |
Bond length (Å) |
Bond length (Å) |
||
|
α |
β |
||||
|
Vacuum |
AT |
N13(A)-O23(T) |
2.951 |
3.015 |
2.936 |
|
N11(A)-N25(T) |
2.878 |
2.869 |
2.891 |
||
|
C1(A)-O24(T) |
3.674 |
3.619 |
3.758 |
||
|
Dihedral angle |
179.88° |
179.07° |
176.16° |
||
|
GC |
N23(G)-O17(C) |
2.934 |
2.964 |
2.909 |
|
|
N9(G)-N18(C) |
2.95 |
2.967 |
2.956 |
||
|
O20(G)-N26(C) |
2.817 |
2.808 |
2.845 |
||
|
Dihedral angle |
179.92° |
179.05° |
179.99° |
||
|
Water |
AT |
N13(A)-O23(T) |
2.962 |
2.959 |
2.945 |
|
N11(A)-N25(T) |
2.921 |
2.916 |
2.942 |
||
|
C1(A)-O24(T) |
3.719 |
3.705 |
3.823 |
||
|
Dihedral angle |
179.98° |
174.97° |
169.97° |
||
|
GC |
N23(G)-O17(C) |
2.88 |
2.931 |
2.886 |
|
|
2.958 |
2.953 |
2.958 |
|||
|
2.909 |
2.895 |
2.9 |
|||
|
179.83° |
178.56° |
179.74° |
|||
Table 2 The structural properties of AT and GC base pair in presence of hydroxyl in vacuum and H2O solvent
The most and least stable structures belong to the GC-OH/β-vacuum and GC-OH/α-H2O cases. Also there was a charge transfer between hydroxyl radical and base pairs. In vacuum, electrons are transferred from hydroxyl radical structure to AT and GC base pairs but in H2O solvent, electrons are transferred from AT and GC base pairs to the Hydroxyl radical.
Theoretical calculation was used in order to investigate the influence of hydroxyl radical on DNA base pairs. The hydroxyl radical increasing the length of the nearest hydrogen bond of adjacent AT or GC base pair, which is accompanied by decreasing the length of furthest hydrogen bond. Also, the dihedral angel between base pairs was changed by hydroxyl radical. These deformations of hydrogen bonds change the natural structure of DNA strands and facilitate the DNA damage.
The authors acknowledge University of Kurdistan for supporting this project. None of the authors have any competing interests in the manuscript.
The authors declare that there is no conflict of interest.

©2016 Zarei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.