Journal of
eISSN: 2373-633X


Research Article Volume 10 Issue 3
1Radiation Oncology Department, Papageorgiou General Hospital, Greece
2Medical Physics Department, Papageorgiou General Hospital, Greece
Correspondence: Efstathios Kamperis, Radiation Oncology Department, Papageorgiou General Hospital, Thessaloniki, Greece, Tel +306976761308
Received: May 18, 2019 | Published: May 24, 2019
Citation: Kamperis E, Kodona C, Hatziioannou K, et al. Dosimetric impact of rotational setup errors in H&N patients undergoing VMAT with respect to clinical target volume coverage and parotid complication probability. J Cancer Prev Curr Res. 2019;10(3):64-67. DOI: 10.15406/jcpcr.2019.10.00393
Purpose: To assess the impact of rotational setup errors on clinical target volume coverage and parotid complication probability in patients undergoing VMAT for head and neck malignancies.
Methods: Twenty eight H&N VMAT plans were retrospectively reviewed. Online automatic registration with respect to bone anatomy was performed using a model with 4 degrees of freedom. Translational errors were corrected as opposed to rotational that were not. Instead, they were simulated via couch rotation at the treatment planning system. The new dose distributions were evaluated and compared against the non rotated ones regarding CTV and parotids irradiation. CTV D98% and conformation numbers were calculated for both plans to assess the impact of rotation on target coverage and healthy tissue irradiated volume. For parotids, a Lyman Kutcher Burman model of normal tissue complication probability for flow rate < 25% was used.
Results: The average rotational error in our cohort was 1.4o (95% CI: 0.9-2.0o). In 7.1% of the cases D98% reduction was at least 2%. When D98% increased, the plan’s global maximum Dmax (0.03cc) also increased by an average 1.9% (95% CI: 0.7-3.1%). In one case the dose distribution was affected so intensely by the rotational shift, that Dmax was displaced off CTV. Conformation numbers deteriorated in the presence of rotational errors. The average difference of CN was -2.0% (95% CI: -3.5, -0.4). Regarding parotids’ NTCP, in 17.9% the absolute gain on mean parotid dose exceeded 1 Gy and the associated increment in NTCP was 4% (95% CI 0.7-7.2%). The mean rotational error on this particular subset was 2.6o (95% CI 0.5-4.8o).
Conclusion: CTV coverage in H&N cancer patients undergoing VMAT may be compromised by rotational setup errors. Their impact on parotids NTCP depends on planned mean parotid dose and the absolute magnitude of rotation. Institutions need to calculate appropriate cut-off values to apply couch rotation, whereas for larger errors repositioning the patient would be more prudent.
Keywords: Rotational setup errors, VMAT, xerostomia, NTCP
The advancement of radiation therapy during last years culminated to the implementation of modern techniques like Volumetric Modulated Arc Therapy (VMAT)1 which constitutes the evolution of Intensity Modulated Radiotherapy (IMRT).2 Both of these treatment modalities have greatly increased our ability to conform dose to target volumes and spare surrounding normal tissues by virtue of steep dose gradients. These intricate dose distributions are susceptible to geometric errors that may compromise tumor coverage and normal tissue complication probability.3,4 A commonplace approach to correct six dimensional setup errors is to move the treatment couch along the three translational axes. Still, a translational shift can only partially counterbalance rotational errors, presumably leaving residual errors behind.5 The situation is further complicated by the contribution of additional uncertainty factors such as weight loss, tumor and organ (e.g. parotid) shrinkage during the course of treatment.6–8
Radiation induced xerostomia is a frequent side effect in the treatment of locally advanced head and neck malignancies with serious ramifications in the patient’s quality of life. Besides the subjective perception of mouth dryness, reduced salivary output may render mastication and swallowing problematic, lead to nutritional deficiencies, dental caries and in its extreme to osteoradionecrosis.9 Sparing of parotid glands with IMRT or VMAT contributes to significant recovery of saliva secretion and improves quality of life.10,11 The purpose of this study is bifold. To assess the impact of rotational setup errors to targets and parotids and to provide cut-off values for repositioning the patient. Clearly, the latter depends on planning margins, frequency of imaging and immobilisation technique.12–15
Twenty eight patients participated in this study. All of them had an histologically confirmed diagnosis of locally advanced squamous cell H&N cancer and underwent VMAT with curative intent. Patients were treated in supine position and immobilized using a 3- or 5-point fixation custom-made thermoplastic mask in a carbon frame along with a kneefix. Target volumes and organs at risk were delineated on the reference CT image according to International Commission of Radiation Unit and Measurement 50 and 62 recommendations.16,17 All planning treatment volumes (PTV) were derived from clinical target volumes with a 3mm isotropic expansion. VMAT plans were constructed in Eclipse Treatment Planning (Version 11, Varian Medical System) using a simultaneously integrated boost technique with three or four dose levels (6996, 6600, 5940, 5445 cGy at 33 fractions). Treatment delivery was performed on a 6MV energy linear accelerator (DHX, Varian Medical System, Palo Alto, CA). For setup verification Varian On-Board Imager® was used.
All patients’ records were retrospectively reviewed. They had received one CBCT scan per week during their course of treatment, except for the first week when three consecutive CBCTs were performed at days 1-3. Online, automatic, intensity-based registration with respect to bone anatomy (C2-C6) was performed using a 4 degrees of freedom model. Translational and yaw rotational setup errors were recorded, but pitch and roll errors were not on the grounds that they cannot be handled by our linac’s couch. Only translational corrections were applied to the treatment couch, provided they were out of “tolerance” which, in our department, is 2mm. Contrariwise, rotational errors are under consideration on how to optimally be dealt with. Options include no action, couch rotation or repeating patient setup.
For every patient the mean rotational error was calculated from the first five CBCT scans. Only the latter were considered in an effort to rule out confounding variables such as weight loss, tumor and organ shrinkage that occur later during the course of radiotherapy. At the treatment planning system, yaw errors were simulated via a couch rotation equal to the mean rotational error of the corresponding patient. Subsequently, the original plan was recalculated based on the rotated CT scan. The new dose distributions were compared against the non rotated ones regarding CTV and parotids irradiation.
Study’s dosimetric endpoints
As far as the CTV was concerned D98% values were compared. Differences less than 2% were considered clinically not significant. Also Dmax of 0.03cc of the treatment plan was evaluated, both quantitatively and visually.
The impact of residual rotational errors on conformality was assessed with Conformation Number by van’t Ri et al.18 As seen in Eq. 1, CN has two components. The first fraction describes the quality of target coverage per Radiation Therapy Oncology Group definition;19 the second fraction describes the volume of normal tissue receiving a dose equal or greater to a reference dose. A value of 1 represents a reference isodose covering the target volume without irradiation of normal tissues and corresponds to optimal conformation. We used the 95% of prescribed dose level for a specified target as the reference dose in order to apply van’t Reit’s formula.
(Eq. 1)
Equation 1. van’t Riet formula for conformation number (CN).
Abbreviations: TV, Target Volume; TVRi, Target Volume covered by reference dose; VRi, Volume of reference dose.
Niemerko proposed a DVH reduction scheme where an organ’s DVH is reduced to a single dose named equivalent uniform dose (EUD). Assuming the entire organ is irradiated uniformly to this particular EUD, the NTCP is the same as the original non-uniform dose distribution.20 The dose response of the parotids is modeled by a sigmoid-shape function, hence a Lyman-Kutcher-Burman model was used to calculate the NTCP for reduced flow rate. The values of the model’s parameters consisted of TD50 (uniform dose resulting in 50% complication probability) equal to 39.9 Gy and m (steepness of curve) equal to 0.40, as per the combined Michigan and Utrecht experience.21 The NTCP was then calculated via the following equations:
Assuming a parallel arrangement for parotids (n=1) the EUD is simplified to:
The statistical calculations were performed with Wolfram Mathematica version 11.3.
The average rotational error in our study was 1.4o (95% CI: 0.9-2.0o) ranging from 0 to 5.3o in good agreement with previous reports.22 Overall, D98% was reduced by 1.7% (95% CI: 2.0, 0.7) in the rotated plan in 28.5% of cases. In 7.1% this reduction was clinically significant, i.e. more than 2%. Conversely, when D98% increased the plan’s global maximum Dmax (0.03cc) also increased by an average 1.9% (95% CI: 0.7, 3.1%) up to 4.5%. Strikingly, in one case the dose distribution was considerably perturbed by the rotational shift, resulting in a Dmax outside of CTV (but still within PTV).
CNs were calculated for both the original and the rotated plan and were found to deteriorate in the presence of rotational errors, as expected. The % difference in CN (ΔCN%) was computed for every patient of the cohort. The relation between mean rotational error and CN degradation is demonstrated in Figure 1. The average difference of conformation number was -1.95% (95% CI: -3.50, -0.41). Both target conformance and volume of irradiated healthy tissue deteriorated, in the rotated plans, by 0.26% (95% CI: -0.29, 0.81) and 2.58% (95% CI: 0.36, 4.81), respectively. Regarding the effect of rotation on parotid sparing, in 17.9% of cases the absolute gain on mean parotid dose exceeded 1 Gy and the associated increment in NTCP was 4% (95% CI: 0.74-7.2%) ranging from 1.7% to 8.4%. The mean rotational error on this particular subset was 2.7o (95% CI 1.1-4.3o) ranging from 0.4o up to 5.3o. Figure 2 shows the % difference in mean parotid dose as a function of rotational error.
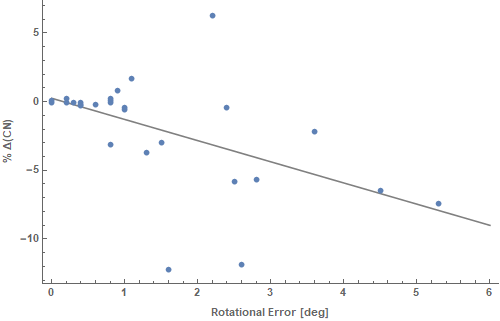
Figure 1 The relation between mean rotational error and CN degradation is demonstrated. CNs were calculated for both the original and the rotated plan and were found to deteriorate in the presence of rotational errors, as expected.
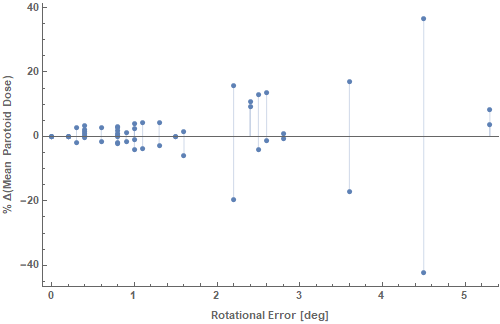
Figure 2 The % difference in mean parotid dose as a function of rotational error is demonstrated. For every rotational error in the horizontal axis, two dose points are shown, one for each of the two parotids of the same patient. Occasionally, the increase in one parotid is associated with a decrease in the contralateral. Yet, one should take into account the planned mean dose in order to calculate the resultant NTCP, i.e. the increase in ipsilateral mean parotid dose may confer a greatest increase in NTCP rather than the respective NTCP decrease of the contralateral parotid.
In this study we retrospectively evaluated the overall setup errors in a head & neck patient cohort. Historically it has been difficult to detect rotations with conventional 2D portal imaging, but nowadays 6D setup errors are effortlessly derived. Although there exist couches with tilting and rolling capability, they are not universally available, may spawn additional uncertainties and require protective measures to ensure patient safety.5 Setup errors consist of two components, a translational one that is corrected online, along with a rotational component that is left uncorrected. There is an abundant literature of studies dealing, from a computational standpoint, with six degrees of freedom variations and their dosimetric effect on treatment plan.23–25 Yue et al.26 made further progress by developing a mathematical formalism that introduces a combination of isocenter shift, gantry, couch and collimator rotation in order to simulate, and eventually correct, full six-degree setup variations.26 Their method, though, cannot be applied to VMAT treatments due to static gantry requirement nor generalized to all IMRT plans due to collision constraints.26
In our analysis we considered only translational shifts and yaw rotations. We estimated the effect of the latter on CTV coverage, volume of healthy tissue irradiated and parotid sparing, by recomputing the dose distribution on the rotated image set. Commercial TPS do not routinely offer image rotation tools, hence this is usually achieved with in-house developed software.23 In other studies roll errors are implemented as gantry rotation.27,28
The dosimetric impact of a head rotation correlates with the distance of the structure of interest from the axis of rotation, increasing as we move towards the periphery of the body. In head and neck cases, parotids are particularly vulnerable to rotation in the axial plane.27 Guckenberger et al.22 recorded rotational errors >2o in 11.1% of head and neck treatments with maximum rotational error 6o, significantly affecting steep dose gradients and elongated target volumes particularly at the targets’ ends.22 In our analysis we also considered the mean parotid dose prior to the rotational drift as a predictor for NCTP degradation, on top of the magnitude of rotation per se. Given our relatively small number of cases though, we could not adequately sample the entire parotid dose-rotational error space (Figure 3).
According to Murphy rotational corrections should always be either applied when included in rigid-body setup calculations or excluded altogether and, in the latter case, registration should be done at the area of target site.29 This is a premise that cannot always be fulfilled at head and neck malignancies since registration landmarks should include C2 to C6 vertebra due to severity of inadvertent spinal cord irradiation.30
In certain cases rotational errors may compromise CTV coverage and dose conformity substantially. Their impact on parotids NTCP depends on the interplay between planned mean parotid dose and absolute magnitude of rotation per se. A cut-off value of 2.5o could be used as a rule of thumb when applying couch rotation, whereas for larger errors a reposition would be more prudent as linac’s geometric constraints become relevant.

©2019 Kamperis, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.