Journal of
eISSN: 2373-633X


Case Report Volume 11 Issue 6
1Department of Medical Oncology, Hassan II University Hospital, Fes, Morocco
2Department of Radiology, Hassan II University Hospital, Fes, Morocco
3Department of Pathology, Hassan II University Hospital, Fes, Morocco
Correspondence: Lamyae Nouiakh, Department of Medical Oncology, Hassan II University Hospital, Fes, Morocco, Tel +212 641510692
Received: December 06, 2020 | Published: December 31, 2020
Citation: Nouiakh L, Oualla K, Ouafki I, et al. Diffuse large cell type B breast lymphoma: case report. J Cancer Prev Curr Res. 2020;11(6):148-152. DOI: 10.15406/jcpcr.2020.11.00444
Background: Primary breast lymphoma is a very rare disease. The diffuse large B cell subtype is the most common histological variety. The clinical presentation is non-specific; it can be similar to that found in breast carcinoma. The diagnosis is only retained after a definitive histological analysis. The management of these lymphomas is no different from that of other localizations. We report the case of primary breast diffuse large B cells lymphoma collected at the medical oncology department of the Hassan II Hospital of Fez in a 32-year-old patient. Through this work, we will discuss the epidemiological, clinical, paraclinical, histological, therapeutic, and prognostic characteristics of these tumors.
Case presentation: A 32-year-old woman consulted for a left breast nodule evolving for 3 months. Clinical examination objectified a painless mass of the left breast without inflammatory signs and homolateral axillary adenopathy of 3cm. Mammography and breast ultrasound were performed objectifying three tissue lesions measuring 26mm for the largest in the left breast, associated with homolateral axillary adenopathy. A micro biopsy was performed. Histological analysis showed breast parenchyma largely dissociated by cell proliferation of diffuse architecture with large cells. In immunohistochemistry, tumor cells were positive for the anti-CD20 antibody. An extension assessment with thoracoabdominal computed tomography did not show any secondary localization outside the breast lesions found in the mammography and ultrasound. Chemotherapy with R-CHOP was decided, but the patient refused to be treated for family reasons.
Conclusion: Through this work, a case of DLBCL was reported with a review of the literature. In summary, the DLBCL histological subtype is the most frequent form of mammary lymphoma. Consolidation radiotherapy after conventional chemotherapy remains the most reasonable therapeutic modality for the treatment of patients with DLBCL. Large studies are necessary to identify the factors that influence survival, to improve the management of patients with these lymphomas.
breast, diffuse large B cell lymphoma, R-CHOP
A 32-year-old patient with unremarkable medical history, uniparous, breastfeeding, presented at the consultation following the autopalpation of a nodule of the left breast evolving for 3months. Clinical examination found a patient in good general status (PS to 1). The senological examination objectified a painless mass, not adherent to the upper quadrant of the left breast without inflammatory signs. The examination of the contralateral breast was normal. Examination of the lymph node areas revealed left axillary lymphadenopathy of 3cm fixed compared to the 02planes.
Following this symptomatology, the patient performed mammography and a breast ultrasound objectifying three tissue lesions measuring 26mm for the largest in the left breast, associated with homolateral axillary lymphadenopathy (Figure 1). A micro biopsy was performed. Histological analysis showed breast parenchyma largely dissociated by cell proliferation of diffuse architecture with large cells, with vesicular nuclei and nucleoli with a poorly limited eosinophilic cytoplasm (Figure 2). In immunohistochemistry, tumor cells were positive for the anti-CD20 antibody (Figure 3). An extension assessment with thoracoabdominal computed tomography did not show any secondary localization outside the breast lesions found in the mammography and ultrasound (Figure 4). Chemotherapy with R-CHOP was decided, but the patient refused to be treated for family reasons.
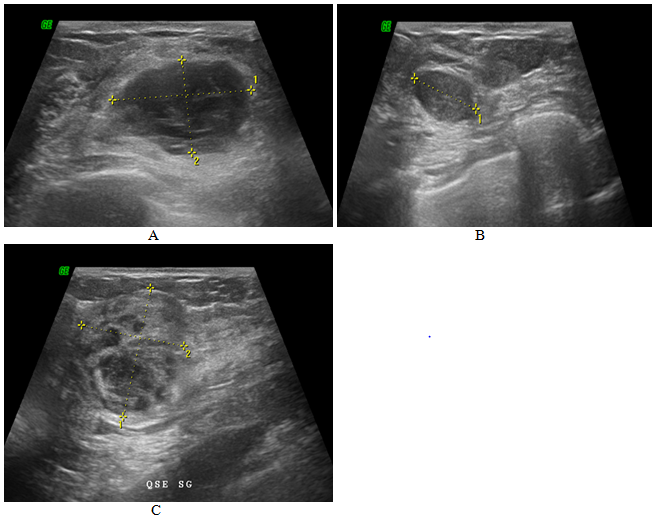
Figure 1 Ultrasound images
(a) Ultrasound section showing tissue mass hypoechogenic in the infero-internal quadrant of the left breast.
(b) Ultrasonographic section showing the lesion of the junction of the external quadrant of the left breast.
(c) Ultrasound section showing tissue mass hypoechogenic in the supero-external quadrant of the left breast.

Figure 2 Histological analysis
(a) HESx100: breast parenchyma infiltrated by lymphoma.
(b) HESx200: breast parenchyma largely dissociated by cell proliferation of diffuse architecture with large cells.
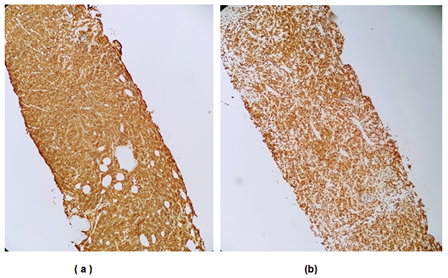
Figure 3 Immunohistochemical analysis
(a) Diffuse labeling with anti-CD20 antibody.
(b) Ki67 estimated at 90%.

Figure 4 Scanographic images
(a) Axial scangraphic section showing lesion in the supero external quadrant of the left breast.
(b) Axial section showing lesion at the infero-internal quadrant of the left breast.
(c) Sagittal scangraphic section showing lesion at the infero-internal quadrant of the left breast.
(d) Sagittal section showing two lesions, one at the upper external quadrant and the other at the junction of the external left breast.
Primary breast lymphoma is an extremely rare pathology, representing 0.04 to 0.5% of breast cancers and 1.7 to 2% of primary extra-lymph node lymphomas.1,2 The rarity of this histological entity is due to the fact that the breast contains a small proportion of lymphoid tissue compared to other organs where primary lymphomas are more frequent.4 The definition of primary breast lymphoma is based on four criteria according to the World Health Organization (WHO) classification of breast tumors, 2003 edition; proposed by Wiseman and his collaborators;5 histological sampling sufficient to make a complete anatomopathological analysis, the association between the breast tissue and lymphomatous infiltration, absence of diagnosis of extra breast lymphoma and metastasis of the disease to expect for homolateral axillary lymphadenopathy.
Diffuse large-cell B lymphoma is the most common histological form, found in 31% of all non-Hodgkin lymphomas. It is a histological subtype that evolves rapidly compared to other histological subtypes.6 This lymphoma is rarely found in men, it usually affects the elderly woman especially around the fifties,7 but it can be observed in young women as the case in our patient. According to the data from the various publications,3,8 the average age of this type of cancer is between 42 and 69 years with extreme ages ranging from 19 to 91 years.
The involvement is often unilateral, most often found in elderly women. Bilaterality is possible, observed in 2 to 16% of cases especially in young, pregnant, or breastfeeding women; it can be synchronous or metachronous.6,9 Bilateral involvement appears to be a characteristic of aggressive disease and poor prognosis. Our patient does not enter into either of the two situations; since she is young, not pregnant, and breastfeeding, having presented a unilateral form. Clinically, it is usually the accidental discovery by the patient or her doctor of a painless breast nodule9 as is the case in our patient. Inflammatory forms can also be found.2 Axillary lymph node involvement is present, found in 20 to 40% of cases, and it should suspect a diagnosis of non-Hodgkin's lymphoma in the presence of bulky lymphadenopathy or the case of unusual localization of these lymphadenopathies.9,10,11
In mammography, there is no specific sign to retain the diagnosis of lymphoma; it is radiologically most often translated into a very limited mass, of homogeneous density evoking a benign tumor without spicular formations or micro calcifications. Rarely, is there a suspicious aspect of imaging malignancy.11 In ultrasound, it is most often a hypoechogenic or even homogeneous anechogenic mass with very limited contours.12 The same radiological presentation was found in our patient. Breast MRI remains superior to mammography and ultrasound; it can detect lymphomatous foci and exclude benign disease. Usually, the lesions are hypotensive in T1 and isointense in T2 with hypertensive halo.10 In our patient, breast MRI was not performed and since the aspect of breast lesions in ultrasound and mammography was not highly suspected of malignancy, but the presence of homolateral axillary lymphadenopathy led to the achievement of a micro biopsy because the definitive diagnosis of lymphoma is retained only on a tissue fragment. Cytological analysis alone does not make the diagnosis. Diffuse large B cell lymphomas are the most common histological form of non-Hodgkin B lymphomas (56-84%) followed by marginal zone lymphomas (9–28%), follicular lymphomas (10–19%) and Burkitt's lymphoma (<6%).13 DLBCL is characterized by a proliferation of diffuse architecture of large cells that have a high mitotic rate. The use of immunohistochemistry makes it possible to make the diagnosis of certainty in the positivity of lymphoid markers.
Therapeutically, there is no univocal therapeutic strategy for the management of patients with DLBCL due to the rarity of these tumors and in the absence of randomized studies. The treatment of DLBCL joins that of other lymphomatous localizations. The R-CHOP protocol (rituximab -cyclophosphamide, doxorubicin, vincristine, and prednisolone) represents the standard regimen for patients with DLBCL regardless of the initial stage. The role of the addition of RITUXIMAB (a monoclonal antibody targeting the CD20 antigen) to poly-chemotherapy was controversial. Very few studies have been published in this sense. According to a retrospective study conducted by Zhao14 comparing 31 patients treated with CHOP with or without RITUXIMAB, the addition of the RITUXIMAB to conventional chemotherapy improved overall survival. However, other retrospective studies did not show this benefit.15 Another study by Avilés & colleagues13 showed that the addition of RITUXIMAB to chemotherapy decreases the rate of relapse at the central nervous system in patients with DLBCL. Regarding the association of radiotherapy with chemotherapy in the management of patients with DLBCL, no formal data is indicating the use of combination therapy in the management of patients with DLBCL. Retrospective studies reported by the Adult Lymphoma Study Group (ALSG) in 2005 and 2007 showed that it was not necessary to combine closure radiotherapy with conventional chemotherapy.16,17 Another retrospective study conducted by G Martinelli & colleagues18 on 200 cases of DLBCL showed a benefit in terms of overall survival and not in progression-free survival of combination therapy. The only randomized trial that proved the benefit of combined therapy in terms of objective response, relapse rate, and overall survival was the trial conducted by Avilés & colleagues13 The results of this study were confirmed by observations made by the International Extranodal Lymphoma Study Group (IELSG).19 So, radiotherapy seems to have a positive impact on the therapeutic strategy of patients with DLBCL, especially in patients without axillary lymph node involvement.
The surgical approach in the overall strategy for the management of DLBCL is not proven.6 Data from the International Extranodal Lymphoma Study Group (IELSG) showed that radical mastectomy was associated with an increased risk of death with HR=2.4 (95% CI 1.2–4.8; P=0,03) in multivariate analysis.19 Therefore, surgery should not be considered a therapeutic modality.
DLBCL are very aggressive tumors, they have two types of relapse, one at the contralateral breast and the other in the central nervous system.5,8,19 The rate of relapse at the brain varies between 5 and 29%, which explains that some authors propose prophylactic intrathecal injections of METHOTREXATE, while others prefer to use it in the presence of neurological signs.20
Through this work, a case of DLBCL was reported with a review of the literature. In summary, primary breast lymphoma is a very rare disease, of which the DLBCL histological subtype is the most frequent form; it is characterized by the non-specificity of clinical and radiological signs whose prognosis remains poor. Consolidation radiotherapy after conventional chemotherapy remains the most reasonable therapeutic modality for the treatment of patients with DLCBL. Large studies are necessary to identify the factors that influence survival, to improve the management of patients with these lymphomas.
None.
The authors declare that they have no competing interests.
The authors received no specific funding for this study.

©2020 Nouiakh, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.