Journal of
eISSN: 2373-633X


Case Report Volume 8 Issue 5
1Medical Laboratory Services Department, Johns Hopkins Aramco Healthcare, KSA
2Pathology Services Department, Johns Hopkins Aramco Healthcare, KSA
3Medical Laboratory Services Department, Johns Hopkins Aramco Healthcare, KSA
4Oncology Services Department, Johns Hopkins Aramco Healthcare, KSA
Correspondence: Colin A Ross, M.D, The Colin A. Ross Institute for Psychological Trauma, 1701 Gateway, #349, Richardson, TX 75080, USA, Tel 438-992-5516, Tel 972-918-9588
Received: October 06, 2017 | Published: October 23, 2017
Citation: Ahmed HA, Amra N, Rabaan A, et al. Denovo BCR-ABL positive acute myelogenous leukemia in adult saudi patient-A case report. J Cancer Prev Curr Res. 2017;8(5):344-346. DOI: 10.15406/jcpcr.2017.08.00293
Background: Acute myeloid leukemia (AML) is a malignant neoplasm of the myeloid lineage. AML is the most common acute leukemia in adults. Its incidence increases with age. Flow Cytometry immunophenotyping is important to differentiate AML from other types of leukemia and to identify the AML subtype. Molecular genetic testing is also an essential part for classification. BCR-ABL positive AML is a rare subtype that is now included as a separate provisional entity in the revised WHO 2016 classification of myeloid malignancies.
Case presentation: A 54-year-old male was diagnosed with AML based on the morphology of the circulating blasts and confirmed by Flow cytometry. Conventional cytogenetics and FISH on diagnosis bone marrow, showed t(9:22) (q34;ql 1.2) and BCR-ABL1 respectively. The patient was treated with standard AML chemotherapy in addition to a tyrosine kinase inhibitor, Dasatinib. Minimal residual disease (MRD) on day a marrow specimen obtained 14 and day 28-post chemotherapy was detected using Flow Cytometry and FISH, which gave comparable results.
Conclusion: to our knowledge, this is the first case encountered at JHAH. We shared our experience and confirmed the validity of MRD detection by Flow Cytometry technique that showed comparative result by molecular technique, indicating a high confidence level of lab testing.
Keywords: acute myeloid leukemia, flow cytometry immunophenotyping, BCR-ABL1 fusion
AML, acute myeloid leukemia; ALL, acute lymphoblastic leukemia; MRD, minimal residual disease detection; FISH, fluorescent in situ hybridization
Acute myeloid leukemia (AML) is a malignant neoplasm of the myeloid lineage. AML is the most common acute leukemia affecting adults; its incidence increases with age.1,2 Its etiology is uncertain. Recurring chromosomal and genetic abnormalities have been identified. AML progresses rapidly and is typically fatal within weeks or months, if untreated.1 It has several subtypes, the prognosis varies among these subtypes.3,4 While a presumptive diagnosis of AML can be made by peripheral blood smear examination under light microscopy, a definitive diagnosis usually requires molecular genetic testing, including chromosomal abnormalities detection by karyotyping, fluorescent in situ hybridization (FISH) and molecular testing for mutations in several genes.1–4 Looking for specific mutations in genes such as FLT3, nucleophosmin, and many others is used to help in predicting the outcome of the disease.5
Flow Cytometry immunophenotyping is important to differentiate AML from other types of leukemia (e.g. acute lymphoblastic leukemia (ALL), and to identify the AML subtype. According to the WHO criteria, the diagnosis of AML is established by the presence of 20% or more myeloid blasts in peripheral blood and/or bone marrow.3,4,6 However the presence of certain cytogenetic abnormalities; t(8;21), inv(16), and t(15;17) is diagnostic irrespective of blast percent. The presence of myeloid sarcoma is also adequate to diagnose AML.4,5
Genetic translocation t(9;22) (q34;ql 1.2), also known as BCR-ABL, is a pre-requisite to diagnose chronic myeloid leukemia (CML) and is the most frequent chromosome anomaly in adult B lymphoblastic leukemia/lymphoma (B- ALL/LBL). De novo BCR-ABL positive AML is a rare subtype that is now included as a separate provisional entity in the revised WHO 2016 classification of myeloid malignancies.7,8
The purpose of this case report is to report a rare case of BCR-ABL positive AML, and to correlate the minimal residual disease detection (MRD) by Flow Cytometry with the gene expression level by molecular technique.
A previously healthy 54-year-old male presented to EMS with dry cough and feeling general unwell. Temperature was 37.4 C. Chest x-ray showed no infiltrate. WBC was 7.2 x 109/L with normal differential, hemoglobin 13 and platelets 154 x109/L. He was felt to have upper respiratory infection. He presented five days having more cough. He was afebrile. There was no hepatosplenomegaly or lymphadenopathy. Chest x-ray showed a right lower lobe infiltrate. White blood cell count (WBC) was 166 x 109/L with 46% neutrophils, bands 5%, metamyelocytes 1%, myelocytes 6% and blasts 27%. Basophils were14%, Hemoglobin was 10.9 g/dL and platelets 140 x 109/L.
Bone marrow aspirate at diagnosis showed hypercellular spicules. About 70% of cells were blasts. There were increased basophils and eosinophils some of which appeared dysplastic with basophilic granules. Bone marrow biopsy had 90-95% cellularity, 80-90% of which was composed of blasts. There was +1 reticulin fiber deposits. Flow Cytometry immunophenotyping showed more than 85% were myeloblasts that were positive for CD45, CD33, CD13, CD117, HLA-DR and aberrantly expressing CD203, CD123, CD22, CD25, CD116 and they lack CD34 antigen (Figure 1).
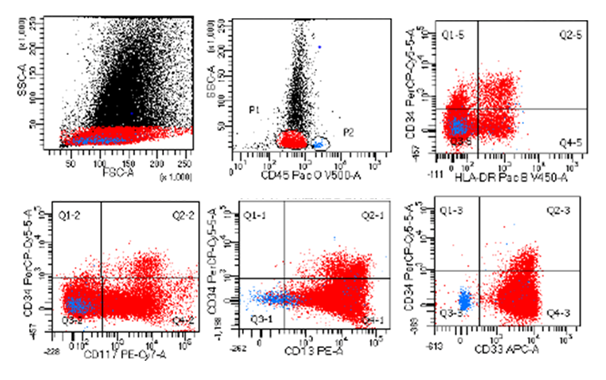
Figure 1 Immunophenotyping dot plots of the diagnostic bone marrow.
Copy Right: These figures were taken from FACSCanto II instrument at Johns Hopkins Aramco Healthcare after IRB committee approval.
AML FISH panel on the marrow showed that 91.4% of nuclei have BCR-ABL1 fusion. No other abnormality was detected. Cytogenetic study revealed that each of 20 metaphase analyzed had 46XY, t(9;22)(q34;ql 1.2). No other abnormality was present. Target for p210 BCR-ABL mRNA was amplified, but quantitation could not be done. Patient was treated with standard chemotherapy for AML with Idarubicin and Cytarabine. When the result of AML FISH became available, Dasatinib was added to the treatment regimen.
A follow up post chemotherapy, minimal residual disease (MRD) on day 14 was detected at 1.62% of total mononuclear cells (cut off is < 0.1%) using FlowCytometry (Figure 2B), and FISH detected BCR-ABL1 fusion in 2.7% of nuclei (cut off is <0.3%). MRD on day 28 post treatment was detected at 0.2% by Flow Cytometry (Figure 2C), and 0.11% by FISH (cut off is <0.067%). BCR-ABL1 mRNA was negative on a peripheral blood specimen on day 42 post induction (Figures 2 & 3).
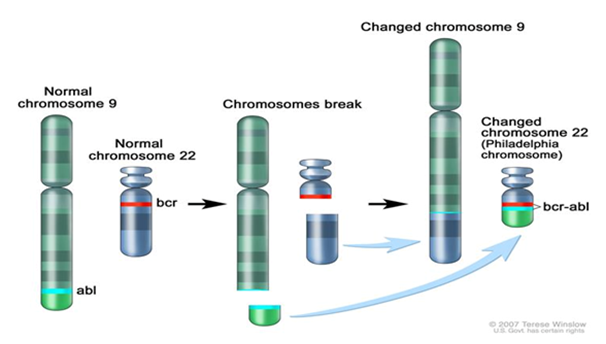
Figure 2 The Philadelphia chromosome is a translocation between the ABL-1 oncogene (on the long arm of chromosome 9) and the breakpoint cluster region (BCR) (on the long arm of chromosome 22), resulting in the fusion gene BCR-ABL1. BCR-ABL1 encodes an oncogenic protein with tyrosine kinase activity.
Copy Right: This figure was taken from National Cancer Institute website at https://www.cancer.gov/types/childhood-cancers/pediatric-genomics-hp-pdq#section/_3
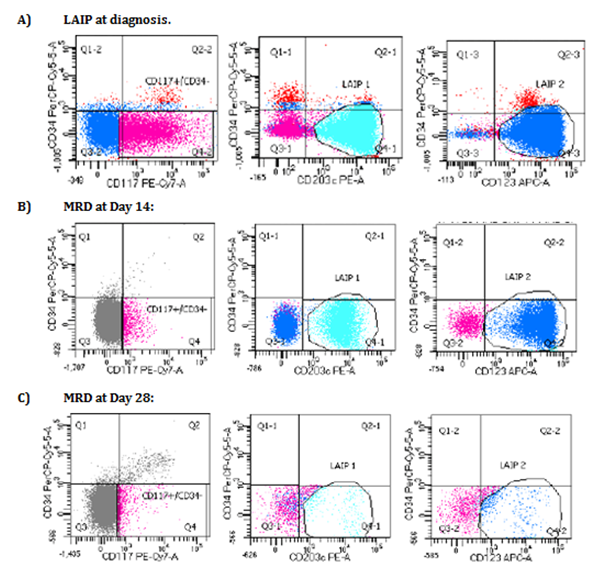
Figure 3 Immunophenotyping dot plots showing the leukemia associated immunophenotype (LAIP) at diagnosis (A), LAIP status in minimal residual disease (MRD) at day 14 post chemotherapy (B), and LAIP status in MRD at day 28 post chemotherapy (C).
Copy Right: These figures were taken from FACSCanto II instrument at Johns Hopkins Aramco Healthcare after IRB committee approval.
The best of our knowledge, this is the first case encountered at JHAH. We herein would like to share our experience. We also have confirmed the validity of MRD detection by Flow Cytometry technique that showed comparative result by molecular technique. This indicates the high confidence level of lab testing using high performance techniques in our institute.9,10
Dr. Nasir Amra
Dr. AdilAlkhatti
Dr. Ali Al Rabaan
Declared, no conflict of interest.
This case report completed after getting the permission from concerned patient.

©2017 Ahmed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.