Journal of
eISSN: 2373-633X


Review Article Volume 2 Issue 3
Internal medicine Department, King Abdulaziz University, Saudi Arabia
Correspondence: Atlal Abusanad, Division of Medical oncology, Internal Medicine Department, King, Abdulaziz university Hospital, Al Sulimanyiah district, P.O. Box 80215, Jeddah 21589, Saudi Arabia, Tel 9.67E+11
Received: January 08, 2015 | Published: March 31, 2015
Citation: Abusanad A. Dendritic cells vaccine: the basics and selected applications in cancer. J Cancer Prev Curr Res. 2015;2(3):54-57. DOI: 10.15406/jcpcr.2015.02.00036
Dendritic cells vaccine (DCV) is a newly emerging and potent form of immune therapy used to treat cancer. DCV enhances the circumvented immune system by educating and reprogramming it to mount a specific immune response against the target. Although this vaccination approach has been effective and feasible, optimal techniques for other aspects of vaccine production are still in the experimental stage. The basic aspects of dendritic cell (DC) biology and functions, along with the DCV preparation process and selected applications are discussed here.
Keywords: immunotherapy, antigen, vaccines, cancer, cell therapy
DC, dendritic cells; DCV, dendritic cell vaccine; PBMCs, peripheral blood mononuclear cells; PAMPS, pathogen-associated molecular patterns; TAA, tumor-associated antigen; MHC, major histo-compatibility; TLRs, toll-like receptors; NK, natural killer cells
Since the discovery of dendritic cells (DC) four decades ago,1 their structure, location, and central role in regulating the immune response have been well elucidated. DCs are highly specialized cells involved in host immune defense against an array of antigenic responses, such as infections of viruses and bacteria, and malignancy. DCs represent an attractive target for immuno-modulation, particularly in diseases such as cancer and HIV infection. The unique qualities and role of DCs in triggering an immune response, combined with the growing evidence that the immune system can attack and eradicate malignant cells and certain chronic infections, form the basis for dendritic cell vaccine (DCV).2
Dendritic cells originate from the bone marrow and represent an extremely heterogeneous population with several subsets defined by their unique cell surface antigens and location. The subsets fall into two main types, myeloid and plasma cytoid, which are derived from monocytes and lymphocytes, respectively.3 Peripheral blood mononuclear cells (PBMCs) have the potential to give rise to DCs under certain conditions, when exposed to pathogen-associated molecular patterns (PAMPS), such as viruses, bacterial lipopolysaccharide (LPS), flagellin, or tumor-associated antigen (TAA), which results in a cytokine-mediated inflammatory response. A small number of DCs are released into the circulation in the immature form (iDC) with the ability to mature in the same fashion as triggered PBMCs.
The DC journey starts once they are released into the circulation, from where they may relocate to a specific tissue or be transported freely in the blood. Some reside in tissue exposed to the exterior environment, functioning as a surveillance apparatus, e.g., Langerhans cells in the skin, where they can be challenged and constantly triggered by the surrounding environment (Figure 1). Similarly, DCs are abundant in the mucosal surfaces of the intestine and airways. Foreign antigen is taken up by the DC, processed, combined with major histo-compatibility (MHC) class II, which, along with self-antigen combined with MHC class I, is presented on the cell surface. They migrate to site-draining lymph nodes and related lymphoid structures guided by chemokine gradients, to present the captured antigen to other cells of the immune system.4 The maturation of DCs is influenced by an array of inflammatory cytokines. Such maturation closely correlates with the nature of the offensive signal.5
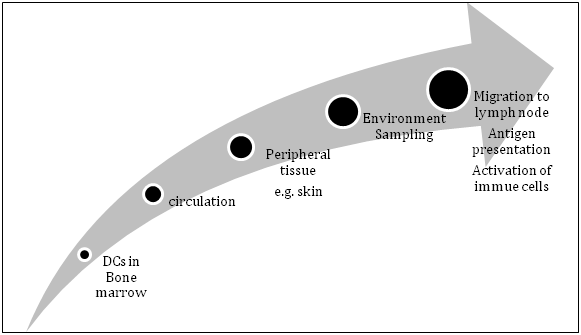
Figure 1 Life cycle of dendritic cells (DCs) from bone marrow to peripheral tissue via circulation where they encounter different Antigens. In the draining lymph node/lymphoid structure, they present captured antigen(s) and interact with other immune cells.
Dendritic cells play a central role in orchestrating the immune response because they:
Dendritic cells vaccine
Dendritic cells vaccine is an active immunotherapy in which antigen-loaded autologous DCs are used to induce an immune response against a specific target. Vaccine production is a multistep process (Figure 2).

Figure 2 Dendritic cells vaccine preparation process. PBMCs, peripheral blood mononuclear cells; iDCs, immature dendritic cells; Ag, antigen
Dendritic cells vaccine preparation
Obtaining dendritic cells: A relatively large number of DCs is required to produce effective vaccines. These are obtained as immature dendritic cells (iDC) from various sources, CD34+ hematopoietic progenitor cells, or CD14+ monocytes precursors.9 Circulating iDCs comprise 0.5 % of PBMCs and can be isolated from T cell- and monocyte-depleted peripheral blood cells after culturing for 48 hours in vitro in the absence of cytokines. While in culture, iDCs become less dense, which facilitate their purification by density-gradient centrifugation.10 Leukapharesis and immuno-phenotyping were used to isolate CD14+ monocytes and blood CD34+ progenitors.11,12
Culturing CD34+ hematopoietic cells or CD14+ monocytes, with a variety of different cytokines in vitro, can generate adequate numbers of DCs. Sallusto et al.,13 were the first to describe the generation of DCs in vitro from CD14+ monocytes after 5–7 days incubation with granulocyte-macrophage colony-stimulating factor (GM-CSF) and IL-4. CD34+ progenitor cells cultured with a combination of GM-CSF and TNF, mature into fully functional DC- like cells.14 The generated cells acquired stable morphologic and functional characteristics of DCs.
In order to minimize the ex vivo manipulation of DCs, and to reduce the risk of contamination, Flt3 ligand was administered in vivo to induce cellular mobilization. Flt3 ligand binds to its tyrosine-kinase receptor (RTK) Flt3 (CD135), which is expressed on early hematopoietic cells, and affects their proliferation. Stimulation of Flt3/CD135 causes an increase in peripheral blood DCs.15 While the optimal method of generating fully functional DCs has not yet been identified, CD14+ monocyte- and CD34+ progenitor-derived DCs are the most widely used and clinically successful.
Antigen selection and loading
A principal element of a successful DCV is identification and selection of the most appropriate antigens. Appropriate antigens for loading DCs are specific, immunogenic, MHC class I- and/or II-avid, stable, and sufficiently expressed by the target. They should also retain their immunogenicity in vivo.
Loading DCs with peptide derived from a known antigen is the most common strategy. Other strategies are the use of whole cells (e.g., apoptotic tumor cells, allogeneic tumor cell lines, and cell lysates), recombinant proteins, immune complexes, and antibodies directed specifically toward DC molecules. Whole cell extracts can provoke a non-specific immune response, but they can also increase the likelihood of immunogenicity because of the presence of polyvalent peptides within the processed cell. However, this approach is less useful in proof-of-principle studies and presentation of self-peptides can result in autoimmunity. In addition, processing such complex antigen could be difficult for DCs with resultant suboptimal antigen presentation. On the contrary, antigen-derived peptide/epitope with or without conjugate may trigger a specific immune response with a reduced risk of autoimmunity but diminished immunogenicity. The limited number of well-characterized antigens and the relatively rapid turnover of exogenous peptides are other significant limitations. The production of an engineered multi-epitope antigen, in an attempt to maximize immunogenicity and specificity while minimizing autoimmunity, may help to overcome these limitations.
Antigen loading strategies such as transducing DCs with a viral vector and transfecting DCs with RNA or plasmid DNA have been tested successfully in animal models.16,17 The goal is to enable a steady and continuous expression of endogenous tumor antigens by DCs, and allow presentation of multiple epitopes with concurrent presentation of both MHC class I- and II-restricted epitopes. However, antigen expression in genetically engineered DCs depends on the transfection efficiency, which is generally low, ranging from 10–20%. Antigen loading techniques can differ according to the type of antigen used in DC priming, but the general practice is to add antigens to DCs in culture and incubate for several days, to enable their recognition and presentation by DCs.
Induction of dendritic cells maturation
Dendritic cells maturation is a process whereby the iDC undergoes several morphologic and functional modifications to become proficient at antigen presentation. It involves the loss of phagocytic and endocytic receptors, upregulation of MHC class II and co-stimulatory/adhesion molecules (CD40, CD50, CD54, CD58, CD80, and CD86), expression of CD83 and chemokine receptor 7 [CCR7] (molecules that are essential for DC migration) and attainment of the characteristic star-like morphology. The mature DC has the capability of producing IL-12, which is required for the development of Th1 cells and the CTL response. In vivo, maturation occurs while DCs are migrating toward the draining lymph node, and is initiated through the activation of TLRs.18 In vitro, a combination of cytokines is used to induce a comparable process. A combination of pro-inflammatory cytokines such as IL-1, TNF-β, IL-6, and prostaglandin E2 was employed and resulted in DCs capable of eliciting potent Th1 cell and CTL responses.19 Culture of iDCs with such as combination of cytokines is currently the gold standard; however, others have also explored TLR-dependent (e.g., LPS, receptor-directed monoclonal antibodies, and immune complexes) and TLR- independent (e.g., RTK stimulation, transfection of RNA/DNA, and exosomes) approaches to induce DC maturation.20
Delivery to the host
Route: As with many aspects of the developmental process of DCV, the optimal route of administration has yet to be identified. Administration routes include intravenous, intradermal, and to a lesser extent intraperitoneal, or directly into the lymphatic vessels and/or lymph nodes.21 Each has its own rationale; intradermal and intravenous routes provide convenience as they are easy to access, but large numbers of DCs are required to ensure that an adequate number reach the lymph nodes to yield the desired effect. The intralymphatic/nodal route provides direct access to the microenvironment where antigen presentation takes place, hence fewer DCs are needed in comparison to other routes, but the need for radiological-guided administration makes it less preferable.
Behind the scenes (migration to the lymph node): The motility and the ability of DCs to migrate toward the lymph node increase with activation and maturation. The migration process is enabled by gradients of chemokines that attract DCs to the site of action. iDCs respond to many chemokines (MIP-1a, MIP-1b, MIP-3a, MIP-5, MCP-3, MCP-4, RANTES, TECK, and SDF-1), which are inducible upon inflammatory stimulation. Mature DCs upregulate CCR7 during activation that has a vital role in the process of migration through the binding of its ligands, CCL19 and CCL21. The latter are produced primarily in the T cell- rich parafollicular areas of the lymph nodes.22 Enriching DC with TNF-α is suggested to optimize the migration process.23 However, little is known about how to optimize DC migration and research is ongoing to optimize DCV delivery.
Response to dendritic cell vaccines
Two interactions have been proposed as mechanisms for the anti-tumor effect of DC-based immunotherapy. The first involves the delivery of DCs directly to the tumor site or the migration of DCs from the vaccine-draining lymph node to the tumor. In the tumor, activated DCs pick up available antigens and produce cytokines creating a pro- inflammatory response, and several antigens are processed and presented by DCs to T cells leading to ‘antigen/epitope spreading’ (an amplified immune response but with relatively low specificity for the vaccine-associated antigen). This response targets the tumor in general. Secondly, DC therapies induce both T cell- mediated helper and cytolytic responses specific for epitopes presented by the transferred DCs. In this model, the anti-tumor effect is mediated via targeting specific TAA.2,3
The capability of DCVs to elicit an immune response in vitro is evaluated with several quantitative and qualitative assays. Cytokine and chemokine production and receptor expression by DCs are measured to assess how mature and competent they are (e.g., IL-12 production is indicative of maturation and CCR7 receptor expression is indicative of migratory capacity). The ability to trigger an immune response beyond a defined threshold is assessed by quantifying the stimulated cells of the immune system (e.g., CD8+ T cells and/or CD4+ T cells).24 In vivo, stimulated T cells in the peripheral blood can be easily accessed for this kind of assessment. However, there is a variable correlation between vaccine parameters and clinical response to vaccination that may be explained in general by the diversity of the host’s immune system, the extent of disease, and the negative immuno modulatory effect of the disease, whether cancer or chronic infection. Disease extent is a recognized factor in predicating the clinical response to DCV; the greater the disease burden, the lower the efficacy. Current evidence, therefore, suggests that vaccination may be best carried out in the adjuvant setting following primary surgery or chemotherapy, when host tumor burden is minimal.
In vivo, a significant obstacle to the success of DC-based immunotherapy is the presence of negative immunomodulation mediated by regulatory T cells, NK cells, NKT cells, and an array of immunosuppressive cytokines (e.g., IL4, IL-6, IL-10, and IFN-γ). Several other mechanisms from the tumor microenvironment inhibit DCs to induce an efficient anti-tumor response. Tumor-derived factors such as VEGF, TGF-β, M-CSF, GM-CSF, IL-6, and IL-10 affect DC functions. For example, TGF-β results in the impairment of DC function, thereby resulting in an accumulation of myeloid-derived suppressor cells, and detrimental M2 macrophages. Down-regulation of such inhibitory immune mechanisms can boost the effect of DCV. In animal models, low-dose cyclophosphamide and depletion of T regulatory (T-reg) and NK cells enhanced the anti-tumor effect of DCV.25
Selected applications of DCV in cancer treatment
Sipuleucel- T is the first approved DCV for patients with asymptomatic or minimally symptomatic metastatic castration-resistant prostate cancer. It is directed against a recombinant antigen (PAP-GM-CSF) that consists of prostatic acid phosphatase (PAP), an antigen expressed in more than 95% of prostate cancers, and GM-CSF, an immune cell activator. It showed significant survival advantage compared to placebo in a phase III trial.26 Certain hematological malignancies such as B cell non-Hodgkin’s lymphomas and multiple myeloma express monoclonal immunoglobulin, which is a readily targetable antigen. DCV directed against the antigenic determinant of immunoglobulin, known as the idiotype (Id) has been used in small clinical trials.27-29 Several melanoma antigens have been identified that bind MHC class I. Among these are gp100, MART-1, tyrosinase, MAGE-1, and MAGE-3. Several early-phase trials showed DCVs against these antigens to be feasible and of therapeutic benefit; however, there is a paucity of information regarding their use in everyday clinical practice.30,31
Dendritic cells are proficient antigen-presenting cells, which have been associated with immunogenicity and tolerogenicity to various tumor- and infection-derived antigens.32,33 They mediate and coordinate the interaction between both innate and adaptive immune responses through their ability to present foreign antigens to different immune cells,7 and possess certain molecular and morphological features that facilitate their key role. DCs work in harmony with other mediators of the immune system and require various cytokines and chemokines to mediate their maturation and migration, and presentation of the offensive antigen.6,18,22
DCs are ideal for immunotherapy in cancer patients. DCVs are made by PBMCs such as lymphocytes or monocytes, harvested from the blood of the patient and grown ex vivo in large amounts while exposing them to antigens (e.g., from the patient’s own cancer).11,12,24 The DC cells with antigens presented on their surface are injected back into the patient. The injected DCs along with the antigens activate the production of CTL and others immune cells, which are programmed to recognize and destroy the cancer cells.3 Several DCVs have been in phase II and III clinical trials.16,26-29 Important considerations in designing DCV trials are: selecting the appropriate antigen, choosing the appropriate cytokine profile to achieve the desired outcome, deciding on the route of administration, and using combinations of treatment modalities. Our knowledge on DC as a valuable tool for exploiting human immunity against diseases in general and cancer in particular is growing apace will be useful in the research for further advances in cancer research.
The author thanks Ms. Lindsey Goff for editing the final draft of this manuscript.
Author declares that there is no conflict of interest.

©2015 Abusanad. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.