Journal of
eISSN: 2373-633X


Research Article Volume 9 Issue 1
1School of Life and Health Sciences, Aston University, England
2Department of Medicine, University of Tennessee Health Science Center, USA
3PNB Vesper Life Science PVT, India
Correspondence: Eric Lattmann, Aston School of Pharmacy, Aston University, Aston Triangle, Birmingham B4 7ET, England, Tel +44-(0) 121 204 3980, Fax 44-(0) 121 359 0733
Received: January 23, 2018 | Published: February 7, 2018
Citation: Lattmann E, Narayanan R, Russell ST, Fleary WA, Singh M et al. (2018) Cholecystokinin Antagonists: Fluorinated 5-Hydroxy-5-Aryl-Pyrrol-2- Ones as Experimental Agents for Brain, Colon and Pancreatic Cancer. J Cancer Prev Curr Res 9(1): 00316. DOI: 10.15406/jcpcr.2018.09.00316
In terms of cholecystokinin-physiology,1 CCK-8 is the most common peptide hormone, which is extensively found throughout the gastrointestinal tract (GIT) and is also widely distributed through the nervous system.2 Originally, cholecystokinin was discovered to cause contractions of the gallbladder.3 It was then rediscovered as pancreozymin, triggering the release of pancreatic enzymes. Finally, it was confirmed that both peptides are identical.4 Cholecystokinin acts as a neuromodulator as well as gut hormone and the cholecystokinin receptor has been extensively investigated as potential drug targets.5 CCK-ligands, agonists and antagonists,6 have been extensively investigated as potential drug molecules.7 They were studied as growth inhibitors in certain forms of cancer,8 as anxiolytics,9 in the treatment of schizophrenia10 and satiety.11 Cholecystokinin does cause proliferation in colon- and pancreatic cancer cell lines and therefore, CCK-antagonists were studied as growth factor inhibitors in certain forms of cancer.
Asperlicin was the first non-peptidal lead structure from nature12 and analogues thereof were studied as CCK ligands.13 Simplification of this lead structure by Merck led to Devazepide,14 a potent CCK1 selective cholecystokinin antagonist (Figure 1), containing a 1,4-benzodiazepine template and an indole moiety. Proglumide15 was the first glutamic acid based agent, marketed as Milid for the treatment of ulcer. Lorglumide, a derivative of proglumide,16 (Figure 1) is one of several CCK receptor antagonists17 and served as experimental standard. The indolyl amide of devazepide was replaced by a urea linkage and Merck’s L-365,260 resulted in a CCK2 selective antagonist.18 Z-360 is a CCK2 -gastrin receptor antagonist and progressed into phase 2 trial with pancreatic cancer.19 Z-360 is the most recent derivative derived from this original lead structure, with improved selectivity and bioavailability.
All structural optimisations did only partly address the main underlying problem with respect to poor pharmacokinetic properties, such as a low water solubility and very low membrane penetration, as a result of a large polar surface area of the molecules and a relatively high molecular weight.
In our early search for new CCK ligands, in which the 1,4-benzodiazepine structure20 was replaced by an achiral diphenyl pyrazolone template, novel CCK antagonists with an indole carboxylic acid21 and a phenyl urea moiety22 were initially optimized as CNS drug with excellent animal data on anxiety and depression.23 The 1,4-benzodiazepine template was varied by a combinatorial solid phase synthesis24 and it was SAR optimized in terms of CCK binding affinity to a benzodiazepine with a simple propyl group.25
Again, having realized the poor pharmacokinetic properties these agents, a search for a completely novel, smaller template with a molecular weight <350, a log p about 3 and a polar surface area for membrane penetration of less that 100A,2 with no urea linkage was initiated.
Aim of the drug discovery programme, initiated by PNB Vesper Life Sciences, was to systematically design from the 2(5H)-furanone scaffold26 a hydroxyl-pyrrolone scaffold with ligands for both CCK1 and CCK2 pathways.
Initial results for CCK antagonists of the pyrrolone scaffold were communicated in the area of cancer therapeutics27 and inflammation.28 Here, a full biological evaluation of the cancer duo PNB-028 & PNB-291 will be reported in detail with respect to their antineoplastic properties.29
Experimental section
.125I-CCK-8 Radioligand cholecystokinin binding assay: CCK2 and CCK1 receptor binding assays were performed, by using guinea pig cerebral cortex or rat pancreas. Male guinea pig brain tissues were prepared according to the modified method described by Saita et al.30 Pancreatic membranes were prepared as described by Charpentier et al.31. Tissues were homogenized in ice cold sucrose (0.32 M, 25 ml) for 15 strokes at 500 rpm and centrifuged at 13000 rpm for 10 minutes. The supernatant was re-centrifuged at 13000 rpm for 20 minutes. The resulting pellet was re-dispersed to the required volume of buffer at 500 rpm and stored in aliquots at 700C.
Binding was achieved using radioligand125I-Bolton-Hunter labeled CCK, NEN at 25 pM. The samples were incubated with membranes (0.1 mg/ml) in 20 mM Hepes, 1 mM EGTA, 5 mM MgCl2, 150 mM NaCl, at pH 6.5 for 2 hrs at RT and then centrifuged at 11000 rpm for 5 minutes. The membrane pellets were washed twice with water and the bound radioactivity was measured in a Packard Cobra Auto-gamma counter (B5005). Binding assays were carried out with L-363, 260 as control.
Isolated tissue preparations: Male Sprague Dawley rats, weighing 200-250g were used and all animal care and experimental protocols adhered to the relevant laws and guidelines of the institution. The animals were housed under standard conditions of temperature (25°C) with unrestricted access to food and water. The animals were sacrificed using cervical dislocation without anaesthesia. From the abdomen of the animals, the duodenum was carefully excised and washed with physiological solution. The mesentery of the tissue was removed and the lumen was gently flushed with Tyrode’s solution to clear luminal contents. The prepared isolated tissue was rapidly incubated in Tyrode’s solution maintained at 320C and gassed with 95% O2 / 5% CO2. Tyrode’s solution was freshly prepared daily (g/l): NaCl, 8.0; KCl, 0.2; CaCl2, 0.2; MgSO4, 0.1; NaH2PO4, 0.05; NaHCO3, 1.0; Glucose, 1.0. The main equipment used was the Radnoti single unit tissue bath system with a chamber capacity of 35 ml. Bath aeration with carbogen (O2 95%, CO2 5%) was maintained at a constant temperature (32°C). The force in grams was measured with an isometric transducer linked to a power lab data acquisition system.
Electrically stimulated muscle contractions: The intramural nerves within the ileal strips were excited by rectangular pulses of 2 ms, 25 mA and a frequency of 0.2 Hz. Transmural stimulation was applied using two platinum electrodes, one placed in the lumen of the ileum and the other outside the tissue.
CCK-5, penta-gastrin and cholecystokinin CCK-8 preparations: CCK-8S was dissolved in distilled water to prepare a stock solution of 500 µM solution, from which cumulative additions of increasing concentrations (0.1 nM, 1 nM, 5 nM, 10 nM, 20 nM, 30 nM, and 40 nM) were tested to plot a dose response curve. Test molecules and lorglumide were added to the organ bath 10 minutes before exposure to the next CCK-8S serial concentrations. The same protocol was used for pentagastrin, CCK-5.
Molecular modeling
For target preparation the protein structures, pdb identifier 1HZN for the CCK1 and 1L4T for the CCK2-gastrin receptor were downloaded from the protein data bank (www.rcs.org) and docking was performed using Autodock Vina and Hex. After several docking trials for the CCK1/CCK2 receptor the results were analysed and visualized using Chimera and Designer studio 4.5. After visual inspection the results were presented to rationalize drug ligand interactions with the each CCK receptor subtype.
Allograft study
In vivo experiments in mice - assessment of anti-tumour inhibition
Pure strain NMRI mice aged between 6 and 8 weeks from our inbred colony were used for transplanting MAC (murine colon cancer) tumours. Animals were fed on RM3E diet (Lillco-England) and water ad libitum. Approximately 2 mm cubes containing 2x 105 cells of MAC 16 tumour fragments were transplanted subcutaneously in the inguinal region via a trocar in a volume of 0.2 ml. Tumour bearing mice were randomised in groups of 7 animals per group and the treatment was started 10 days after transplantation. The test compounds were administered in propylene glycol. The effect of chemotherapy was assessed 20 days after transplantation. Mice were killed after 10 days of drug treatment and the effects were measured by the differences in tumour weight as expressed: %.inhibition = Treated weight control weight x 100.
The body weight changes were recorded additionally. The procedure was approved by the home office and the bioethics committee of Aston University.
Xenograft study in NSG mice
1 million cells were used per mouse and the test molecule was administered in 20% DMS0 + 80% PEG-300. The suspension was vortexed and warmed at 37 oC for 5 min to ensure dissolution. MAC 16 mouse chemo resistant colon cancer cells were grown in RPMI medium supplemented with 10% fetal bovine serum (FBS) in 37oC incubator with 5% C02. Human cancer cells were grown in DMEM supplemented with 10% FBS in 37oC incubator with 5% C02. Cells were grown until 70% confluence before passaging into fresh flasks. For xenograft implantation, above indicated cells were harvested, viable cells determined by trypan blue exclusion, and a cell suspension in growth medium was prepared. The cell suspension in growth medium (100-111/mouse) was implanted subcutaneously in NSG mice. Once tumours reached 100 mm3, the animals were randomized within the respective cell line and treated orally. Body weight and tumour volume were measured thrice weekly (Mon, Wed, and Fri). Animals were sacrificed (6 hrs after the last dose) when the tumours reached over 1500 mm3 or when the animals lost over 20% body weight.
All experiments were performed in compliance with the relevant laws and institutional guidelines and the institutional bioethics committee has approved the experiments.
PK analysis
6-8 week male rats purchased from Harlan Research Laboratories, North America Registration Number: Syngene-IAEC-412-08-2013 aged 6 to 8 weeks old Identification : They were identified individually with tail marking using permanent marker Acclimatization : At least for one week under laboratory conditions, after veterinary examination. Only animals without any visible signs of illness were used for the study.
Time points for blood sampling (IV dose) were pre-dose, 0.083, 0.25, 0.5, 1, 2, 4,6, 8, & 24 hr post dose (10 time points). Time-points for blood collection (PO dose) was, pre-dose, 0.25, 0.5, 1, 2, 4, 6, 8, & 24 hr post dose (9 time points). At each time point, approximately 150μL of blood was collected through jugular vein in labeled tubes containing K2-EDTA as anticoagulant. The tubes were mixed gently and centrifuged at 2500 g for 10 minutes at 4°C. The plasma was separated into labeled polypropylene tubes (~75μl of plasma) and stored immediately at -80°C until analysis.
Analysis of samples by LC-MS/MS was done using API Sciex 4000 system operated with Nexera™ UHPLC (Shimadzu) as front-end. Samples were separated on a Phenomenex kinetex C18 (50X2.1 mm, 5µ) using a gradient mode at a flow rate of 1 ml/min. The mobile phase consisted of 0.1% formic acid in MilliQ water (A) and 0.1% formic acid in acetonitrile (B). MS instrument was operated in positive mode. The multiple reactions monitoring transition of test molecule was 247.9/192.0 (Q1/Q3) with a declustering potential of 70V, entrance potential 10 V, and collision energy of 25 V. The curtain gas (5 V), ion-spray voltage (5500 V), temperature (500°C), nebulizer gas (GS1), and auxiliary gas (GS2) were set at 45 psi & 55 psi respectively, and the interface heater was on.
The data were expressed as mean + SD and one-way analysis of variance (ANOVA) and supplementary Tukey test for pairwise comparison were tested to determine for any significant difference at p< 0.05.
SAR optimisation
The first step was to screen for potent binding affinity and to identify a CCK1 or CCK2-selective ligand for subsequent in vitro and in vivo evaluation. Using radiolabelled iodinated cholecystokinin, inhibition of binding was determined for all test molecules and the IC50 are outlined in Table 1. Lorglumide served as CCK1 standard and L-365,260 was used as CCK2 standard. The ring enlargement from a 3- membered to a 5- membered ring system (entry 11-12) resulted in no significant increase of binding affinity.
Lactam |
X= |
R= |
CCK1[mM] |
CCK2[mM] |
7 |
H |
Isobutyl- |
0.020±0.01 |
1.2±0.3 |
8 |
Cl |
Isobutyl- |
0.008±0.01 |
0.4±0.2 |
9 |
F |
Isobutyl- |
0.012±0.01 |
0.75±0.2 |
10 |
H |
t-butyl- |
0.12±0.22 |
0.9±0.03 |
11 |
H |
Cyclopentyl- |
0.36±0.03 |
0.84±0.2 |
12 |
Cl |
Cyclopentyl- |
2.5±0.03 |
>10 |
13 |
H |
Hexyl- |
4.5±0.3 |
>10 |
14 |
Cl |
Hexyl- |
3.6±0.3 |
>10 |
15 |
H |
Cyclohexyl- |
2.5±0.3 |
>10 |
16 |
H |
Ph- |
>10 |
>10 |
17 |
H |
Bz- |
0.85±0.03 |
0.020±0.005 |
18 |
Cl |
Bz- |
0.51±0.004 |
0.022±0.004 |
19 |
F |
Bz- |
0.11±0.004 |
0.012±0.004 |
20 |
F |
p-F-Bz- |
0.080±0.004 |
0.018±0.004 |
21 |
H |
Methylbenzyl- |
0.6±0.04 |
0.021±0.01 |
22 |
H |
Methylbenzyl- |
0.42±0.03 |
0.21±0.05 |
23 |
H |
Phenylethyl- |
>10 |
0.022±0.002 |
24 |
Cl |
Phenylethyl |
>10 |
0.030±0.001 |
Lorglumide |
0.17±0.01 |
>10 |
||
L-356,260 |
|
|
0.25±0.01 |
0.003±0.001 |
Table 1 CCK binding affinity expressed in IC50 in micromolar using iodinated hot CCK8 as radioligands with cortex and pancreatic membranes; N=3
The change from N-propyl into the N-butyl group resulted in a manifold increase of activity and the best substituent on the central nitrogen atom was found iso-butyl, as seen for derivative 7. The introduction of a halogen atom into the para- position of the phenyl group resulted in an increase of binding affinity as observed in lactame 8 and lactame 9. Lactame 9 of equipotent binding affinity was obtained as crystalline material in better yields than the chlorinated analogue. The iso-butyl group on the N-atom produced the best overall ligand, with CCK1 selectivity and the IC50 was reduced significantly for t-butyl derivative 10. The n-pentyl analogue was formed in very low yields and the n-hexyl lactames 13, 14 clearly lost binding affinity and the same was observed for the cyclohexyl derivative 15.
Lactames, such as pyrrolone 16, containing an aromatic ring, directly connected to the N-position, showed a micromolar activity > 10mM. Most interestingly, the introduction of an N-benzyl substituent to the pyrrolone template, instead of an alkyl group, resulted in non-selective CCK-ligand, pyrrolone 17. Again, halogen atoms, such as chlorine, only marginally changed the binding affinity for the chlorinated N-benzyl lactame 18. The introduction of fluorine resulted in a significantly improved binding affinity, thus providing us with a mixed CCK antagonist.
A second para fluorine in the benzyl position did not enhance binding affinity any further, but due to inhibition of para hydroxylation, resulted in a molecule with manifold oral bioavailability, lactame 20. The introduction of a chiral amine, such as methyl benzyl amine provided diastereoisomers (Entry 21, 22), which were separated by column chromatography, and both stereoisomers occurred a lower affinity than the parent benzyl derivative 17.
The introduction of a spacer, a single CH2 group, resulted in a phenyl-ethyl derivative 23, which represented a highly CCK2 selective ligand. Lactame 23 is 450 times selective for the CCK2 / gastrin receptor. Halogenation, the introduction of a para- chlorine atom on the phenyl–position, lactame 24, did not enhance binding affinity any further; possibly due to drug receptor interaction of the phenyl group with a lipophilic cavity within the CCK receptor.
Overall, the introduction of alkyl groups, most preferred an isobutyl- group, provided a CCK1 selective antagonist a hydroxyl-pyrrolone 9, which was the selected development candidate PNB-028 for colon and pancreatic cancer associated with the CCKC receptor.29
The N-benzylated pyrrolone 17 displayed a non-selective receptor binding profile and served as lead structure for optimisation. The benzylated fluoro-analogue 19 served as optimised molecule for preclinical pharmacological evaluation as PNB-091 until bioavailability was taken into consideration at a later development stage (Figure 2).
Molecular modelling studies were performed for isobutyl derivative with the CCK1 receptor (Figure 3a).
The isobutyl group of the ligand interacted with a hydrophobic cave of the receptor, centred at Ala-14. The carbonyl group in the 2-position bond via hydrogen binding towards the CCK receptor with Arg-9 and the N- atom of the lactam interacted with Glu-17. The 5-hydroxy- group of the ligand displayed interactions with of Asn-6, while the phenyl group has no interaction with tryptophan or phenylalanine. Pi - alkyl interactions only may explain the small increase in binding affinity of the chlorinated analogue of PNB-081, based on interaction with Leu-29 and Ile-28. If the para phenyl position is not blocked by the chorine atom, most interestingly, the proposed metabolite can interact with the CCK2 receptor. It is supposed that lactam 7, is hydroxylated in the para phenyl position by P450 and this metabolite may interact with the CCK2 receptor via His 122 interaction. Most interestingly hydroxylation may also enhance CCK1 affinity due to interactions with Arg 9.
The docking of lactame 23 into the CCK2 receptor is outlined in Figure 3b and some key interactions are highlighted for one final pose of minimal energy (Figure 3b).
The 5-hydroxy group of the central pyrrolone template interacted via hydrogen binding with the N group of Trp114. The phenyl group of the N-phenylethyl- side chain bound to the aromatic indole system of Trp114 and electron withdrawing groups may enhance these aromatic interactions. The lipophilic pocket allowed principally a wide range of substituents, but only phenyl and not cyclo-hexyl could be realised synthetically. The 5- phenyl group of the pyrrolone template bound via Ile 184 and Leu 133, based on van der Waals interactions and not aromatic interactions. The introduction of electron withdrawing groups, such as halogen atoms, is therefore not enhancing affinity and optimisation on this side is with limited effect. Overall, the observed binding affinity and the predicted interaction based on molecular modelling interaction correlated well for the cholecystokinin and the gastrin receptor.
Isolated tissue preparations: from ligands to antagonists
Opiate agonists, such as morphine and CCK antagonists, such as lorglumide and devazepide reduced electrically induced contractions on the GPI. From the radioligand binding assay the pyrrolones were identified as potent ligands, and the classical isolated tissue preparation served as initial functional assay, confirming the antagonistic properties of these ligands.
Using the isolated rat duodenum preparation, stable amplitude was generated and a reduction of this amplitude was observed dose dependently for lactame 19, which is outlined in Figure 4. This assay represents a fast and efficient way to screen for CCK antagonists using classical isolated tissue preparations. PNB-091 acted as much lower concentrations than lorglumide, the CCK standard.
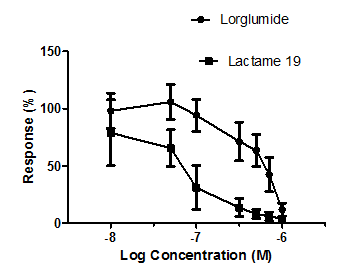
Figure 4 Concentration - response curves of lactame 19, PNB-091 and lorglumide on electrically induced contractions of the rat intestine. Values are mean ± standard error of mean, n = 3. Vertical lines represent error bars.
Cholecystokinin, CCK8s, induced contractions of the guinea pig gall bladder and this second tissue based assay was applied to reconfirm with this standard preparation the antagonistic properties of the potent CCK ligands (Figure 5).
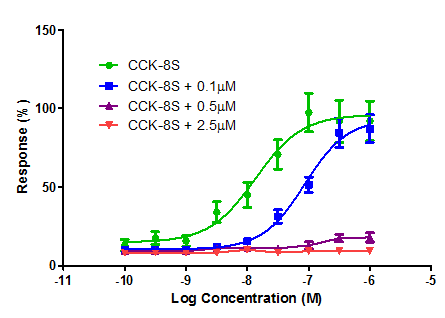
Figure 5 Log concentration-response curves obtained in the presence of CCK-8S alone and CCK-8S in the presence of lactame 19.
CCK-8s induced contractions of the gall bladder and rat duodenum these contractions were reduced dose dependently, which is outlined for the mixed CCK pyrrolone 19 in Figure 5. It appeared that the effect of the antagonist in the rat duodenum assay is insurmountable and an irreversible inhibitor is ideal for the development of antineoplastic agents.
The function of the fluorine atom in PNB-091 was found dual to firstly enhance binding affinity and secondly to block metabolism of the molecules in the para - phenyl position, thus enhancing oral bioavailability.
From 24 molecules in Table 1 the isobutyl substituent and the benzyl group on the N were recognized essential and 2 series of close analogues were screened in vitro using cell based assays.
CCK antagonists are associated with an array of therapeutic applications, but the focus of our research programme was to provide a non-toxic orally available CCK antagonist for the treatment of cancers. The antineoplastic properties of selected NCE and standard CCK antagonists, such as L-365,260 (CCK2), devazepide and lorglumide (CCK1) were subsequently investigated; using a range of CCK associated cancer cell lines.
In vitro tests were originally performed using a cell count assay, as the aim was to inhibit proliferation and not to optimise cytotoxicity, but the results were identical in the methylthiazolyltetrazolium (MTT) assay, possible due to induced apoptosis. It was screened for an inhibitor of viability in certain CCK related cancers cell lines using the MTT assay.
Miapaca is a human pancreatic carcinoma cell line and selected data are outlined in Table 2. Pyrrolone 9 was found 20 times better in terms of IC50 concentrations than lorglumide. Devazepide, which gave an IC50 about 1 µM showed agonistic activity (unpublished results). Lorglumide, a CCK1 antagonist, was acting on the gallbladder CCK1 receptor and not the pancreatic CCK receptor. None of the known CCK antagonists, devazepide or lorglumide proved to be clinical useful, which is in line with our results.
IC50 [nM] |
MIA PACA |
PANC |
MAC13 |
MAC16 |
U373MG |
SHSY5Y |
U-87 |
L-365,260 |
>5000 |
>1000 |
>5000 |
>5000 |
541±87 |
441±54 |
841±88 |
Devazepide |
1200 |
480±32 |
1100 |
1000 |
>5000 |
987±87 |
562±87 |
Lorglumide |
425±23 |
316±21 |
421±11 |
861±22 |
>5000 |
>5000 |
>5000 |
Pyrrolone 7 |
15±4 |
27±9 |
536±32 |
96±25 |
765±29 |
>5000 |
>5000 |
Pyrrolone 8 |
13±4 |
22±9 |
526±34 |
86±23 |
475±25 |
>5000 |
>5000 |
Pyrrolone 9 |
11±4 |
18±6 |
508±35 |
76±20 |
665±28 |
231±21 |
141±11 |
Pyrrolone 17 |
230±11 |
674±33 |
>1000 |
>1000 |
23±3 |
18±7 |
14±8 |
Pyrrolone 19 |
230±11 |
674±33 |
>1000 |
>1000 |
8±4 |
11±2 |
18±6 |
Pyrrolone 20 |
230±11 |
674±33 |
>1000 |
>1000 |
20±4 |
13±4 |
15±4 |
Table 2 Cytotoxicity assay, IC50 of selected examples against a variety of GI and brain cell lines. IC50 values are based on inhibition of viability in the MTT assay in various cell lines
The PANC cell line is a human pancreatic cell line. Pyrrolone 9 is many times better than devazepide / lorglumide on this selected pancreatic cell line. Cancer treatment is combination therapy, even for hormone dependent cancers. For this pancreatic cell line the synergistic effects of MK-329 = devazepide in conjunction with cisplatin were reported.24 and studies to investigate pyrrolone 9, which is now PNB-028 with cis - platin and 5-FU are ongoing.
Overall, pyrrolone 7-9 were not significantly different from each other and the parent pyrrol 7 and the fluorinated pyrrolone 9 were tested subsequently in vivo in xenograft models under consideration of their bioavailability. In vitro in cell based assays they were found of equal selective toxicity towards 2 human pancreatic and 2 colon cancer cell lines. The cytotoxicity results for CCK related cell lines are outlined in Table 2 with lorglumide and devazepide as CCK1 standard and L-365,260 as CCK2 standard.
The antineoplastic effect, mediated by blocking CCK receptors, which are expressed in parts of the GI system, correlated well in vitro cell culture and isolated tissue preparations. MAC 13 and MAC 16 cancers are derived from the colon of the mouse and MAC 16 is of particular interest, as it is resistant to alkylating agents.
For pyrrolone 7, an IC50 about 100 nM was determined for the MAC 16 cell line and the fluorinated analogue 9 was slightly more potent. Most interestingly, no other CCK antagonist, such as L-365,260, devazepide or lorglumide was found active for this cell line, which is, when transplanted into mice lethal within weeks. Therefore, it may be concluded, that the unsurmountable irreversible properties of the antagonists are key to colon cancer.
The anticancer activity of the agents is not limited to GI cancers. The cytotoxicity of the glioblastoma cell line U373MG, a brain cancer cell line, was determined for a small series of test compounds including for Merck’s CCK2 antagonist L-365,260. The CCK1 antagonist, pyrrolone 7 and L-365,260 were found of high nanomolar activity. The mixed CCK antagonist, benzylated pyrrolone 17 showed low nanomolar activity and key was to improve the oral bioavailability. The fluorinated pyrrolone 19 with enhanced receptor binding affinity provided a molecule with best in vitro activity on this humane brain cancer cell line. The introduction of a second fluorine atom for pyrrolone 20 furnished a molecule with similar binding affinity and cell based activity, but due to inhibition of the hydroxylation site, a 3 fold oral bioavailability was obtained.
A mixed antagonist may work best for treating brain cancers, as mengionomas express CCK1 receptors and astrocytomas CCK2 receptors.25 A human glioma cell line, U-87 responded to CCK2 antagonists,.26 occurred higher IC value in contrast to SHSY5Y is a further neuroblastoma human cell line reduced level of CCK expression. Most remarkable for this cell line the best inhibition of growths was obtained cell based, indicating possibly the blockage of other non-CCK pathways.
Subsequently, this tumour was studied initially in mice to analyse, if the in vitro results could be translated into relevant anti-tumour activity.
Colon cancer in vivo study
MAC 16 tumours developed quickly within 6 days after implantation and grew robustly. Once the tumours reached 100 mm,3 the animals were divided into the various treatment groups and treated with vehicle or the fluorinated pyrrolone 9. Iso-butylated pyrrolones inhibited the tumour growth significantly from the day treatment was initiated and maintained tumour inhibitory activity until sacrifice. At sacrifice, interestingly in some animals growths were not only inhibited, but tumours shrinked after reaching a maximum volume. Most interestingly, while MAC 16 tumours in the vehicle-treated group metastasized to lungs, tumours in drug-treated groups did not metastasize to lungs (Figure 6a & 6b).
The control showed nearly exponential growths for this aggressive tumour. The treatment group by oral administration at optimum level (100 mg/kg) showed strong inhibition of tumour growths and the regiment study with bi-daily instead of daily dosing intervals (24h-48h) showed enhanced activity and no signs of toxicity. The analysis of body weight showed an expected loss of 20%, which lead to termination at day 7 and for the 48h regimen an increase of body weight was found.
Cholecystokinin is known to mediate cancer progression and metastasis, which were antagonised by sufficient concentrations of devazepide.27 Colon cancer is generally linked with the CCK1 receptor and only under certain conditions it is associated with the CCK2 receptor.28 Gastric cancer is correlated with the CCK2 receptor / gastrin receptor and in the human gastric cancer cell line HT-29, gastrin-17 stimulated growths and theses effects were abolished by CCK2 antagonists such as L-365,260. In detail Colucci tested only one 1 nM concentration of devazepide, which stimulated growths in the study and by our own observations. Low nanomolar devazepide concentrations occur in vitro a CCK agonistic effect and this may explain why devazepide showed no significant effect in cancer previously.12
Overall for the MAC 16 cell line proliferation was blocked in vitro and in vivo by our pyrrolone based CCK antagonist PNB-028. Here, the link CCK1 receptor and colon cancer was confirmed experimentally.
Efforts to elucidate the underlying anticancer action were made and no significant apoptosis and no influence on angiogenesis by angiogenesis marker CD34, was observed. Proliferation, analysed by proliferation marker KI67, was performed and the results were outlined in Figure 7.
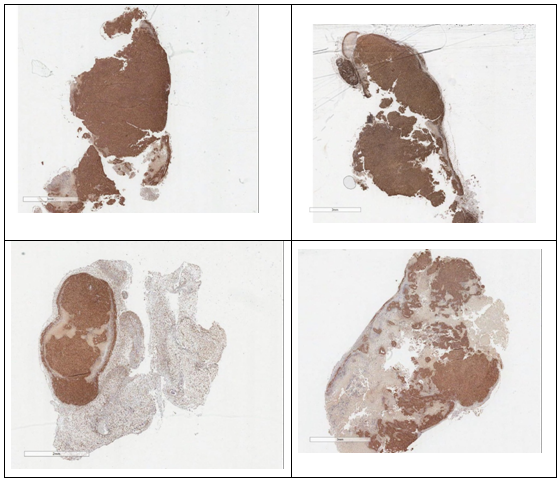
Figure 7 Histology. Ki67 staining: top control, bottom PNB-028 treated 50 mg/kg by PO administration.
For the minimal dose of 50 mg/kg in mice, the tissue was stained (TOP) and in the treatment group large white areas of proliferation free tissue was seen.
These findings are particularly important for the understanding of the standard colon cancer biomarker CEA. Dell death, as a result of apoptosis caused by Pt agents and antimetabolite 5-FU resulted in an increase of biomarker CEA, followed by a decline from week 4 onwards in man.
In the previous communication initial data on pancreatic cancer was shown, and here, under the consideration of oral bioavailability a study was performed in immunosuppressed mice under consideration of oral bioavailability.
For lactam 7 the parent hydroxyl-pyrrolone 7 an oral bioavailability was determined as 24% in rats for the 10mg/kg dose. Pyrrolone 9, assigned as PNB-028 had 98% oral bioavailability and represents the CCK1 antagonist with the highest known bio-availability of all published molecules. The analysis of the plasma concentration in rats was analysed for lactam 7 (24%) and is outlined in Figure 8a.
The selected benzylated pyrrolone 19 (Figure 8b) showed 15 % oral bioavailability for the 10mg/kg dose in rats and further SAR optimisation was achieved by inhibiting metabolism in the para hydroxyl position. The introduction of a second fluorine atom had little influence on binding affinity, but enhanced bioavailability to 34%.
All relevant molecules are outlined in Figure 9 and a SAR optimisation with respect to metabolism was elucidated. The isobutyl group, as aliphatic side chain, is inert towards metabolism and the initial bioavailability for the pyrrolone 7 is medium. After the introduction of a fluorine atom a nearly full oral bioavailability was achieved for this molecule PNB-028. This CCK antagonist occurred the best bioavalability compared to present competitors and will enable us the achieve plasma concentrations to shut down CCK mediated cancer growths pathways.
The benzylated pyrrolone 17 had initially a poor bioavailability of 1.7% and first fluorination nearly enhanced bioavailability nearly 10 fold for 19, PNB-091 to 15%. A second halogenation inhibiting the hydroxylation of the N-benzyl substituent on the para position resulted in a further 2 fold increase (lactam 20), giving a 34% oral bioavailability. Fortunately, the fine chemical 4-fluoro-benzylamine is slightly more costly than the industrial benzyl-amine, thus overall reducing the costs of production of the fully optimised molecule 20, which entered preclinical development as PNB-291 for the treatment of brain cancers.
MIAPACA tumours developed quickly after implantation and grew modestly, though not as robustly as MAC16 tumours. Once the tumours reached 100 mm,3 the animals were divided into various treatment groups and were treated with vehicle or respective drugs. Though vehicle-treated MIAPACA tumours grew from 100 mm3 to over 750 mm3 within 3 weeks, pyrrolone treated tumours grew slower. Based on bioavailability, the fluorinated isobutylpyrrolone 9 was administered at 20 mg/kg and the parent isobuyl prrrolone 7 at 60 mg/kg and after initial faster action the fluorinated pyrrolone 9 at the equivalent dose reduced total inhibition of tumour growths above 90%.
Both agents inhibited tumour growth significantly from the day treatment was initiated and maintained the growth inhibitory properties until sacrifice (Figure 10).
Overall, it was clearly shown, that colon and pancreatic cancers response to CCK1 antagonists and not CCK2 antagonists.29 We agree, it is unlikely that, gastrin is more important in pancreatic cancer than cholecystokinin. It may be wise to say, that it is pancreozymin, via the pancreatic cholecystokinin receptor, that promotes cancer (CCK1b = pancreozymin receptor and not CCK1a cholecystokinin receptor) or possibly the CCK-C receptor. Smith clearly stated the role of the CCK-C receptor in pancreatic cancer.30 and reviewed the cholecystokinin receptor as molecular target in treating pancreatic cancer.31 It is the CCK-C receptor, then according to recent nomenclature -the CCK3 receptor- would be the cancer target and molecular modelling based on the whole receptor, generated by homology modelling would be the starting point for further optimisation towards the CCK3 receptor. This would be advantageous as the CCK1 receptor is required for the physiological role of cholecystokinin. Blocking of the contractions of the gall bladder leads to gallstones in the long term and reductions of bile flow will result generally in liver toxicity.
The benzylated pyrrolones 17, 19 and 20 are mixed CCK antagonists, based on receptor binding confirmed by isolated tissue preparations. In vitro in cell based assays they inhibit proliferation of selected cancer cell line, such as U373MG, SHSY5Y and U-87.
The cell lines differ in CCK expression and the selected cell line in vivo has some expression of the cholecystokinin receptor. The fluorinated pyrrolone 19 was chosen for in vivo evaluation using a humane cell line transplanted into immunosuppressed mice.
The fluorinated isobutyl pyrrol 9 has some interesting in vitro activity on cell lines, but for the fluorinated benzyl pyrrolone 19 the lowest IC50 was determined on all 3 selected humane brain cancer cell lines. As a model substance PNB-091 was selected for further in vivo studies using nude mice and the results are outlined in Figure 11a & 11b.
The transplanted human brain tumour cell line proliferated exponentially and 50 mg/kg reduced growths significantly and a further reduction was found dose dependently.
No weight loss, an indicator of toxicity was observed, the vehicle was not significantly different even from the highest administered dose and no visual signs of toxicity were found in all mice for the duration of the experiment.
According to bioavailability the bis-fluorinated pyrrol on 20 would be efficient at below 30 mg/kg and this was proofed using MAC16 tumours in mice (not included). 65% nhibition of brain cancer is significant and 30mg/kg in mice would be the equivalent to a 200 mg capsule in man.
These novel fluorinated pyrrolones PNB-028 and PNB-091/PNB-291 were synthesised in only 2 synthetic steps in high overall yields from generally readily available, inexpensive starting material.
Molecules were identified classically in radiolabelled binding assays and SAR was fully optimised for a small series with in vitro cell based assays and tissue based assays. SAR in terms of pharmacokinetics was considered at a very early stage and in vivo evaluation with normal and nude mice resulted in 2 molecules for colon & pancreatic and a non- selective antagonist for the treatment of brain cancers.
For this novel template, it was found, that irreversible CCK antagonism translated into anti-cancer activity on the gut-brain-axis. The known CNS activity of CCK antagonists may be of additional therapeutic benefit in treating cancer -associated diseases, such as pain, anxiety and depression.
The experimental work was partly supported by PNB Vesper Life Sciences.
No conflict of interest is declared.
The animal studies were performed in compliance with relevant laws and institutional guidelines.

©2018 Lattmann, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.