Journal of
eISSN: 2373-633X


Literature Review Volume 11 Issue 3
Department of Biochemistry, Adekunle Ajasin University, Nigeria
Correspondence: Oluwaseun Fapohunda, Department of Biochemistry, Adekunle Ajasin University, Akungba Akoko, PMB 001, Nigeria, Tel +2347062998896
Received: May 24, 2020 | Published: June 22, 2020
Citation: Fapohunda O, Ajayi DC. Cancer cell metabolism resulting in multidrug resistance to chemotherapy and possible ways out. J Cancer Prev Curr Res. 2020;11(3):64-70. DOI: 10.15406/jcpcr.2020.11.00429
Cancer has posed a great challenge to its victims all over the world. Researchers have developed several measures to combat this problem. However, it has been discovered that the major hindrance to breakthrough in this field is that cancer cells are able to resist treatment. A large pool of information was retrieved from several reputable articles most especially the recently published ones. In this review, some of the mechanisms adopted by these cells to confer resistance to treatment will be discussed. These mechanisms include drug efflux via ABC transporters, deregulation of cell death mechanisms, modification of drug targets and repair of damaged DNA. The possible means to overcome this resistance are also reviewed.
Reports have shown that cancer is a serious menace to human life and health.1,2 Over the years, it has become a global threat to the human race. Cancer, a group of diseases which is a serious challenge to humans, is usually characterized by uncontrolled cell growth, morphological and cellular transformation, angiogenesis, deregulation of apoptosis, metastasis, abnormal growth or division of cells (neoplasia).3–6 A collection of cancer cells is often referred to as a “tumor” and the terms “tumor” and “cancer” may be used interchangeably.7 Globally, asides cardiovascular diseases, a high mortality rate can also be attributed to cancer (WHO, 2018). Based on a study by Globocan-2018, it was reported that there were 18.1 million new cancer cases and 9.6 million cancer deaths in 2018.8 Deaths associated to cancer increased by about 17% in comparison to data available in 2012. Lung cancer is reported to be prevalent among males, while breast cancer is the most common in females.9 It is expected that the number of new cases will reach 23.6 million by 2030.1,2 Cancer therapies that has been adopted include: surgery, radiotherapy and chemotherapy which is the most common.2,10
Lately, even though chemotherapy drugs have been effective in treating cancer patients, multidrug resistance to drugs by these cancer cells has posed a great challenge.2,11–14 Cancer cells develop measures which enable them to inhibit drugs thereby reducing the drug’s effectiveness. The potency of chemotherapy drugs, which has been limited by multidrug resistance, is a major threat in the treatment of cancer.15,16 Generally, drug resistance can either be endogenous (intrinsic) drug resistance or acquired drug resistance.2,17,18
Different mechanisms of multidrug resistance (MDR) which include dysregulation of drugs transporters, defects of apoptosis and autophagy machinery, alterations of drug metabolism and drug targets, etc., have been identified but their mode of actions is not yet clear.13 Due to the limitations in the known cancer treatments, researchers are now working on new methods to overcome multidrug resistance.16,19
Multidrug resistance (MDR) is a phenomenon characterized by the ability of cancer cells to confer resistance or to become tolerant to antitumor drugs using several mechanisms.6,17,18 MDR was first observed in bacteria as they became resistant to some antibiotics, but it has been seen in some other cases and also cancer.20 This resistance can either be natural (intrinsic) if tumors become insensitive to therapeutic agents before the commencement of treatment or acquired when tumor becomes resistant during the course of the treatment.21,22 Chemotherapy is one of most important treatments for various cancer entities.
Multidrug resistance in cancer has been attributed to the expression of transporters that eject drugs from cells (drug efflux) reducing the efficacy of the drugs.18 Other mechanisms involved in multidrug resistance may include: deregulation of cell death mechanisms, modification of drug targets, damaged DNA repair, decreased intracellular accumulation of anticancer drugs, etc.16
The identified mechanisms of multidrug resistance in cancer can act on their own or by combining with different signal transduction pathways. Some of these mechanisms include:
Drug efflux through ABC transporters
One of the largest known protein families is the ABC transporters. These transporters contain several groups of active membrane transporters with special functions. The human ABC protein family has 49 ABC proteins which are divided into seven subfamilies from ABCA to ABCG.23 The transporters on the plasma membrane major in the influx and efflux of drugs, and they are associated with drug resistance.24 These groups of protein families have the ability to reduce the amount of drug that reaches the drug target by pumping out the anticancer drugs out of cancer cells after administration of drugs thereby leading to multidrug resistance.25 Determination of drug resistance is made possible by these transporters as they regulate the absorption of drugs, their distribution, metabolism and their elimination (ADME). The ABC transporters also limit membrane permeability through the blood-tissue barrier (BTB) and blood-brain barrier (BBB), especially ABCB1 (ATP-binding cassette subfamily B member 1, which is also referred to as P-glycoprotein; P-gp), ABCC1 (that is multidrug resistance associated protein 1) and ABDG2 (that is breast cancer resistance protein).26,27 Overexpression of these transporters are involved in MDR in different types of cancer. The overexpression of ABCB1 confers MDR to some anticancer drugs, including paclitaxel, etoposide, teniposide, doxorubicin, actinomycin D, sunitinib, and tacrolimus.28
As it has been shown that overexpression of ABC transporters leads to efflux of chemotherapeutic drugs, therefore, inhibiting these transporters is a means to overcome drug resistance. Modulators that can inhibit these transporters are being designed, enabling antitumor drugs to reach the drug targets.29 Chemosensitizers such as cyclosporine A and tariquidar, which are ABCB1 and ABCC1 inhibitors were tested in clinical trials.30 However, their ability to overcome drug resistance in cancer treatment is yet limited.29,31 Recently, new methods are being developed to limit the overexpression of ABC transporters, some of which include drug delivery systems, siRNAs and microRNAs delivered by nanoparticles, and so on (Figure 1).25,30,32
Deregulation of cell death mechanisms
The effectiveness of anticancer drugs majors on their ability to cause cell death. Anticancer drugs can cause cell death by inducing reactive oxygen species (ROS), DNA damage and activation of pro-apoptotic receptors.34,35 A major challenge to this is the ability of cancer cells to constantly evolve which enables them to escape cell death.36 Cell death mechanisms include apoptosis, necrosis, and autophagy.37
As earlier stated, anticancer drugs initiate cell death by increasing the levels reactive oxygen species. Glutathione (GSH) is a major cellular antioxidant defense system and is involved in several metabolic processes. The overexpression of GSH leads to drug resistance by decreasing reactive oxygen species levels, this prevents DNA damage and enhances DNA repair.35,39 It was reported by Staudigl et al.40 that high ɤ- glutamyltransferase (one of the enzyme that metabolizes glutathione) levels were correlated with therapy resistance in patients with breast cancer. Glutathione S-transferases (GST), a super family of enzymes that conjugates GSH to chemical toxins, is another example. These enzymes help to repress oxidative stress in the human body. It has been shown that overexpression of GST influences cancer survival.41–46
DNA damage repair
Cancer stem cells (CSCs) have a unique ability to repair DNA damage, this enables them to preserve their DNA. This DNA damage repair mechanism enhances multidrug resistance in cancer cells.48,49 Repair mechanisms used may include nucleotide excision repair (NER), mismatch repair (MMR),base excision repair (BER) and double-strand break (DSB).47 Dysregulation of these repair systems may be involved in multidrug resistance in cancer cells.50 Bonannoet al.51 reported that chemotherapeutics containing platinum e.g. cisplatin, cause harmful DNA cross links and lead to apoptosis. However, nucleotide excision repair and homologous recombination which are the primary DNA repair mechanisms involved in reversing platinum damage enable cancer cells develop resistance to drugs containing platinum.20,51 Thus, the effectiveness of anticancer drugs depends on the inability of cancer cells to repair DNA damage caused by these chemotherapeutics. The efficacy of the therapy could therefore be increased by inhibiting of repair pathways.
Some of the means to overcome the tolerance of cancer cells to anticancer therapy include:
Dysregulation of MicroRNA
MicroRNAs (miRNAs) are small non-coding RNAs that regulates a large variety of target genes and expression of proteins at a post-transcriptional level. They are about 20-25 nucleotides long. They down-regulate gene expression at a post-transcriptional level, but may sometimes can activate mRNA translation.52 Peng & Croce53 reported that microRNAs are involved in cancer development, metastasis and multidrug resistance. MicroRNAs have the ability to influence most mechanisms of drug resistance. They modulate chemotherapeutic drug resistance by the regulation of ABC membrane transporters. It was reported by Zhao et al.54 that ABCB1 (P-glycoprotein) is down-regulated significantly by the up-regulation of miR-138. BCL-2 is an essential anti-apoptosis protein and it is targeted by some miRNAs while regulating drug resistance. An et al.13 reported thatmiR-30a suppresses stress-induced autophagy by inhibiting the expression of beclin, an important protein in autophagy.55 Chronic myelogenous leukemia (CML) cells are sensitized to anti-cancer drugs (e.g. imatinib) by up regulating miR-30a. Another means by which miRNAs influence the sensitivity of cancer cells to anticancer drugs is by altering DNA repair pathways, disenabling DNA damage repair (DDR) mechanisms in cancer cells. MiR-182 was reported to down-regulate the expression of Breast cancer type 1(BRCA1) in breast cancer. Ashomologous recombination–mediated DNA repair was impaired, this increased the sensitivity of tumor cells to anticancer drugs such as poly (ADP-ribose) polymerase(PARP) 1 inhibitor.56 According to a study by Sun et al.57 miR-9 could down-regulate BRCA1 and hinder DNA damage repair in ovarian cancer. This enables cancer cells to be sensitive to anticancer drugs such as cisplatin and inhibitors of Poly (ADP-ribose) polymerase (PARP).13
Inhibition of the ABC transporters
ABC transporters are reported to be linked up with the level of chemotherapy and the progression of drug resistance.23,58–62 The overexpression of ABCB1 (P-gp) contributes greatly to drug resistance in cancer cells. P-gp was reported to reduce the efficacy of its substrate drugs by transporting drugs directly out of cancer cells.27 To counter this challenge, efflux function of ABC transporters can be inhibited, thereby reversing drug resistance in cancer cells.23
Midostaurin is a multi-targeted protein kinase inhibitor that has been investigated for the treatment of acute myeloid leukemia (AML). Hsiao et al.27 revealed that midostaurin has been approved recently by the Food and Drug Agency (FDA) and the European Medicines Agency(EMA) for the treatment of adult patients with newly diagnosed fms like tyrosine kinase-3 (FLT3)-mutated AML. It inhibits the transport and drug efflux function of ABCB1 (P-gp) selectively. This enables ABCB1-overexpressing cancer cells to respond to chemotherapeutic drugs, thereby reversing drug resistance. In the treatment of drug resistant cancer cells, it was proposed that midostaurin may be combined with other anticancer drugs for improved efficacy. However, further clinical investigations could be carried out to evaluate its efficacy. Also, tepotinibisan anticancer drug undergoing phase 2 clinical trials. Wu et al.63 reported that tepotinib reverses the effect on ABCB1-mediated drug resistance by interacting with the drug-binding domain of ABCB1. Another example is selonsertib that inhibits Apoptosis signal-regulating kinase 1 (ASK1 is involved in certain diseases, including cancer) selectively, hence, overcoming multidrug resistance in cancer. It interacts with the substrate-binding sites of both ABCB1 and ABCG2. Combining selonsertib with other anticancer drugs could be a way to overcome multidrug resistance in cancer.
Bioconjugation therapy
As tumor cells now confer resistance against certain cancer treatments, making them ineffective in cancer treatment, researchers have conjugated drugs with different molecules and delivery vectors to combat such problems and enhance the therapeutic effect anticancer drugs. This is referred to as bioconjugation therapy. Bioconjugates are the new therapeutic strategies, they have the ability to combat the complications being produced by chemotherapeutics.8 This technique involves delivering drugs via nanocarriersin which the drug associated with the cancer is targeted into the cancer sites. Bioconjugation consists of linking two molecules, usually through a covalent bond, and at least one molecule should be of biological origin.64 Alternatively, they can be peptides,65,66 glycoproteins,67 aptamers,68,69 or interferons,70 etc.; all these have anticancer properties. Bioconjugates are able to target pathological sites and deliver therapeutics to the targeted sites. They also increase the bioavailability of the therapeutics.71 Various bioconjugates are linked by chemical functional groups (Figure 2).
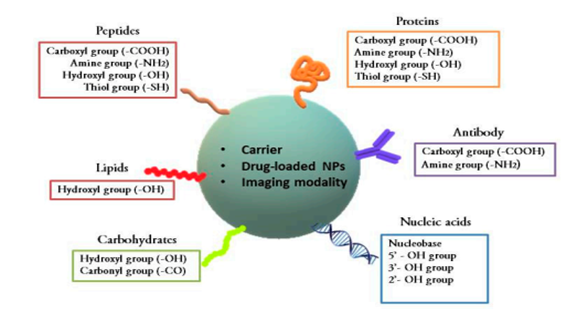
Figure 2 Chemical linkages in bioconjugates. Nanoparticles are abbreviated as NPs.8
Silencing RNA (siRNA) therapy is another measure for cancer treatment. This involves linking antibodies and ligands chemically with siRNA nanoparticles. It is referred to assiRNA-mediated silencing (RNAi) of genes. Oncogenes are genes that has the potential to cause cancer, they are usually mutated or overexpressed in cancer cells. They lead to tumor proliferation, metastasis, angiogenesis, and multidrug resistance. Silencing RNAs target oncogenes and inhibit apoptosis.72 A major problem to the use of bioconjugates is its production cost which is quite expensive, making it unsustainable in underdeveloped and developing countries, especially to the less-privileged.8
Treatment with natural products
A large variety of chemical substances with great importance and usefulness are found in nature. Natural products are molecules with several usefulness, some of them serve as alternatives to synthetic compounds for the development as chemosensitizers. They are combined with chemotherapeutic agents to enhance their efficacy in cancer cells. Synthetic multidrug resistance drugs are limited in treating cancer patients. Current researches have focused on beneficial effects of compounds from natural sources. Several phenol-containing compounds (such as flavones, phenolcarboxylic acids, ellagitannins, stilbens, lignans, curcumin, etc.) and phytochemicals act as chemopreventive agents. They possess antioxidant activity, they inhibit of proliferation, survival and metastasis, and they also modulate immune and inflammatory responses.6
Curcumin, an active constituent of turmeric, is one of the natural products with unique biological activities. It can prevent the initiation of cancer, repress proliferation, and initiates apoptosis in cancer cells. Combining curcumin with other anticancer drugs may sensitize cancer cells to anticancer drugs, this could help to overcome drug resistance in cancer treatment. However, curcumin has been limited in its antitumor function due to its instability and rapid metabolism. In a study by Lopes-Rodrigues and colleagues a small library of new curcumin derivatives were screened for anticancer and ABCB1 regulatory activities. One of the newly synthesized derivatives of curcumin was shown to possess more potent anticancer activity and also has the ability to modulate the function of ABCB1 than curcumin.
Caffeic acid is a non-flavonoid phenolic acid commonly found in vegetables and fruits. It is an antioxidant of biological origin. Due to its antioxidant properties, it has been shown to have anti-inflammatory properties. Teng and co-workers revealed in their study that caffeic acid counters multidrug resistance in cancer. It performs this function by inhibiting the efflux function of human ABCB1 and retains anticancer drugs inside the cancer cells, thus cell death is promoted. Asides this phenolic acid, caffeic acid phenethyl ester (CAPE), a derivative of caffeic acid, possesses some therapeutic effects in the treatment of cancer.
Steroids are used as anti-swelling agent in cancer treatment,73 β-Sitosterol (a plant-based sterol) also possess anticancer activity. Andima et al.74 encapsulated this phytosterol into poly (lactide-co-glycolic acid) (PLGA) and poly (ethylene glycol)-block-poly (lactic acid) nanoparticles. This increased the solubility and therapeutic efficacy of the plant-based sterol against breast cancer.
Previous studies revealed that vitamin D (a fat-soluble vitamin) may inhibit oncogenesis or tumorigenesis and slows down tumor progression. It has anti-inflammatory, immunomodulatory, proapoptotic, and antiangiogenic properties and may decrease death rate from cancer.75–77 It was reported that cancer patients with higher 25-hydroxyvitamin D levels might live longer.78 It was also shown by Chang et al.79 that tenulin (a major sesquiterpene lactone component isolated from Helenium amarum) and isotenulin inhibit the drug efflux function of ABCB1 via inhibition mechanisms through ATPase stimulation. These compounds also restored the sensitivity of cancer cells to chemotherapeutic drugs.80–108
Nil.
The authors declare that they have no competing interests.
No funding source supported this study.

©2020 Fapohunda, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.