Journal of
eISSN: 2373-633X


Review Article Volume 10 Issue 3
Department of Biochemistry & Pharmacology, San Juan Bautista School of Medicine, USA
Correspondence: Yamixa Delgado Reyes, Department of Biochemistry & Pharmacology, San Juan Bautista School of Medicine, Caguas, PR, USA
Received: March 22, 2019 | Published: May 6, 2019
Citation: Delgado Y, Torres A, Milián M. Apoptosis’ activation associated to BH3 only domain and BCL-2 homology domain proteins: new way to design anticancer drugs. J Cancer Prev Curr Res. 2019;10(3):54-59. DOI: 10.15406/jcpcr.2019.10.00391
Cancer is one of the leading health problems we face today. A possible way to develop specific treatments is through the identification and understanding of proteins responsible for the regulation of apoptosis. Apoptosis is a cell suicide that is critically important for organ development and tissue turnover.1 The BCL-2 homology domain (BHD) and BH3-only domain (BOD) are pro-apoptotic proteins that mediate mitochondrial damage, inducing intrinsic pathway cell death. This review wants to explain how BOD and BHD proteins trigger mitochondrial events associated to apoptosis to develop treatments that can promote apoptosis in cancer cells.
Highlights: apoptosis, BH3-only domain, BCL-2 homology domain
Apoptosis or programmed cell dead is a genetically controlled mechanism that plays a crucial role in regulation and maintaining tissue homeostasis leading the elimination of old or damaged cells. Cancer is a group of diseases that appear when abnormal cells growth out of control dividing too quickly invading and destroying adjacent tissues. For these reasons, control of the pathway of apoptosis could help to find or improve treatments for a lot of diseases, especially cancer. Decrease or increase in apoptosis, plays a crucial role in a great many diseases because unhealthy cells become immortal or on the contrary, healthy cells are indiscriminate killed, respectively.
The cell regulates apoptosis via intrinsic and extrinsic pathways. Pro-apoptotic ligands interact with pro-apoptotic receptors, activating the extrinsic pathway apoptosis. To induce intrinsic pathway cell death, BH3-only domain (BOD) proteins interacts with pro-apoptotic BCL homology domain (BHD) proteins to trigger mitochondrial permeabilization and finally, apoptosis (Figure 1). BCL proteins have essential roles on determining if a cell lives or dies because they either promote or block the apoptosis pathway. We found on the literature two groups of BCL family (Figure 1A): the anti-apoptotic members, and the pro-apoptotic members that include the BHD and BOD.

Figure 1 Apoptosis by BH3 domain-only (BOD) proteins in a mitochondrial intrinsic pathway. A. Legend of the anti-apoptotic and pro-apoptotic proteins. B. (i) BOD protein directly binds and activates mitochondrial-localized BAK and BAX, triggering BAK/BAX oligomerization and apoptosis. (ii) BOD promotes apoptosis by binding antiapoptotic BCL-XL protein. (iii) Overexpression of BCL-XL in cancer cells may sequester BOD proteins and thereby block cell death. C. The dephosphorylation of BIM promotes its activation. The BAX/BAK-BIM complex sends the stimuli to permeate the mitochondrial membrane. Cytochrome c is release from the mitochondria activating the caspases and finally killing the cell.
Anti-apoptotic and pro-apoptotic proteins
In the pro-survival or anti-apoptotic group we found BCL-2, BCL-XL, Bfl-1/ A1, BCL-w, MCL-1, Boo, NR-13, BHRF1, LMW5-HL, ORF16, KS-BCL-2, E1B-19K, and CED-9. In the pro-apoptotic group, we also have two groups: BHD (ie, BAX, BAK, NOXA, PUMA, BIK and BAD) and BOD (ie, BIM and BID).2 Both pro-apoptotic BHD and anti-apoptotic proteins share four similar structural domains and a C-terminal membrane anchor. The other pro-apoptotic BOD proteins form amphipathic α-helices and as their name suggests, has only one conserved domain. One of these α-helices interacts with BHD’s active site promoting mitochondrial membrane damage.
A convergence of genetic and biochemical studies has established that in the intrinsic apoptotic pathway, BOD proteins display selective binding to specific BHD family members. These BOD proteins integrate diverse apoptotic stimuli acting at an upstream point in an apoptotic signal-transduction cascade that leads ultimately to cytochrome C release from mitochondria and activation of caspases-9 and caspases-3.3
Intrinsic and extrinsic pathways of apoptosis
Apoptosis is a tightly regulated and at the same time highly efficient cell death program which requires the interplay of a multitude of factors. Apoptotic cells can be recognized by stereotypical morphological changes: the cell shrinks, shows deformation and loses contact with its neighboring cells. Its chromatin condenses and marginates at the nuclear membrane, the plasma membrane is blebbing or budding, and finally the cell is fragmented into compact membrane-enclosed structures, called 'apoptotic bodies' which contain cytosol, the condensed chromatin, and organelles. At the same time, internally biochemical features of apoptosis result from cellular degradation precipitated by intracellular cysteine proteases (caspases), which cleave substrates after specific aspartate residues. These proteases are present in healthy cells as zymogens with low, yet significant, enzymatic activity. In response to apoptotic signals, they become fully activated through adaptor protein-mediated aggregation, which facilitates autocatalytic processing, or through proteolysis by other caspases already activated.4
Mitochondrion is the central regulator of intrinsic apoptosis pathways. On the contrary, the extrinsic pathway begins on the bilayer membrane cell. The activation of extrinsic pathway apoptosis in the type II cells come from the activation of dead receptors (DR) that are on the cell surface. These DR emit apoptotic signals when bound to their specific ligands. The family of the receptors is composed of TNFR-1, Fas/CD95, and the TRAIL DR-4 and DR-5.5 When the receptor recognizes their ligands, it starts a trimerization and activation of the receptor. The cytoplasmic part of the DR sends the signals inside the cell, activating caspase 8. In the case of intrinsic apoptosis, through mitochondrion permeabilization, these cells harbor the capacity to induce caspase 3-activation. In this pathway, pro-apoptotic and anti-apoptotic proteins help to initiate mitochondria-dependent apoptosis (Figure 1A). BOD BIM is the key to links caspase cascade and mitochondrial damage (Figure 1B). The activated form of BIM induces the oligomerization of BAX on the mitochondrion and simultaneously BID sequesters BCL-XL. However overexpression of BCL-XL promotes the binding of BIM and BID proteins, preventing the BAX/BAK oligomer and inhibits apoptosis. If the mitochondrial membrane is depolarized, multiple cellular changes associated with the apoptotic program are initiated, eg, cytochrome c is released, apoptosome is formed and the cascade of caspases is activated via their proteolytic activities (Figure 1C).
There has been quite some debate about how the BCL-2 family controls apoptosis. Commonly, the activation of apoptosis is regarded to occur when a cell encounters a specific death-inducing signal such as the ligation of a death receptor by its ligand. This is the same that occurs in the cell when pro-apoptotic proteins BOD are activated and the interaction with BHD proteins or with BCL-XL, initiate or block apoptosis, respectively. BHD and BCL-XL display sequences conservation in all BH domains, all of which include α-helical segments. BAX activation is believed to be a highly regulated by the contact with BOD, multi-step process involving an interaction-triggered conformational change, mitochondrial translocation, and oligomerization of BAX and BAK that ultimately leads to mitochondrial dysfunction.6 Anti-apoptotic BCL-XL binds directly to the pro-apoptotic members BOD, neutralize their activities, and abolish the apoptotic signalling.7 In healthy cells, inactive BAX and BAK exist as monomers. When BAK and BAX are inactive, they reside at the mitochondrion. In response to dead stimuli, a conformational change occurs to BAK and BAX promoting their oligomerization attached into the mitochondrial membrane.
BOD proteins have an amphipathic BH3 α-helical domain that it is required for dead activation. BOD cannot initiate apoptosis without BH3 sequence because this domain is necessary to bind with BAX (Figure 2). BOD BIM and BID proteins bind to BAX and BAK as well as pro-survival BCL-XL.8 On the contrary, BHD BAD and NOXA BOD preferred to bind anti-apoptotic proteins. BOD family proteins are regulated by phosphorylation and its retention in the cytosol when they receive survival signals. Different studies demonstrate that BIM, BID and BAD could not induce apoptosis in the absence of BAX or BAK.9,10 Using an NMR technique, Gavathiotis et al.,6 determined that the activation site of BAX when it interacts with BIM is at the α1 and α6. Other researchers utilized multiple binding assays, including yeast two-hybrid, co-immunoprecipitation from detergent-solubilized cell lysates, and in vitro pull-down experiments, to indicate that individual BH3-only molecules display some selectivity for multidomain BCL-2 members.11–15 Some studies reveal that BOD BAD is the most prominent in demonstrating the capacity to overcome BCL-XL protection of mitochondria. BAD itself cannot activate BAK or release cytochrome c, probably because BAD sensitize mitochondria to BID or BIM by successfully binding to BAX.16 Additionally, BCL-2 inhibitors may directly induce apoptosis in angiogenically active endothelial cells in vitro, in similar fashion to their effect on tumor cells, and endothelial cell-specific apoptosis can disrupt neovascularization in vivo.17 In vivo studies show that both BIM and BID are the most potent pro-apoptotic proteins inducing BAX/BAK oligomerization, membrane permeabilization, cytochrome C releasing and cascade caspases’ activation, killing human leukemia cells.18 The cytochrome C release occurs by distinct mechanisms that are either Ca+2-dependent or Ca+2-independent. Previous studies validated that Ca+2-independent cytochrome C release seems to be governed by different members of pro-apoptotic BHD, especially oligomeric form of BAX permeabilizing the outer mitochondrial membrane.19 Another important factor is P53 that induces apoptosis by up-regulating expression of the NOXA and PUMA BOD, doing so in response to substantial levels of DNA breaks and other chromosomal abnormalities.20 In addition, a decrease in interleukin-3 or insulin-like growth factor in epithelial cells can induce apoptosis through activation of BIM. All of these concepts show that cancer cells have a plan to disrupt and stop the activation of apoptosis to preserve the growth, proliferation invasion, and the vascularization of the tumor. Our objective is to explain the correlation between cancer and proteins involved in apoptosis pathway; and how we can take advantage of this process to kill cancer cells without affecting healthy cells.
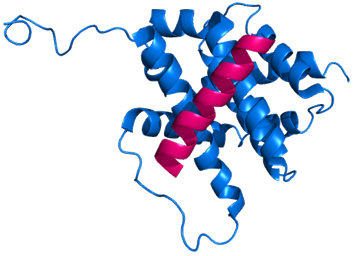
Figure 2 BIM BH3-only domain (showing only conserved α3) (fuchsia) interacting with BAX protein (blue) at α1 and α6. BAX induces cell death when it is activated by the α-helical BH3 domain of BIM. PDB code: 2K7W. Generated using Pymol.
Cancer
When damaged cells acquire the capability to proliferate with uncontrolled growth, a mass of cancer cells develop. Elucidation of the signaling circuitry governing the apoptotic program has revealed how apoptosis is triggered in response to various physiologic stresses that cancer cells experience during the course of tumorigenesis or as a result of anticancer therapy.20 The cells induce apoptosis when they are exposed to oxidative stress, or when increase levels of oncogenes (P53) and/or DNA mutations occur. When tumors are growing the cells are very resistant to apoptotic stimulus and insensitive to proteins that control cell division. In this review, we are focusing on the intrinsic pathway, anti-apoptotic BCL-XL, pro-apoptotic BHD BAX/BAK and pro-apoptotic BOD BIM/BID to explain the apoptosis’ initiation as a viable manner to stop the uncontrolled growth of cancer cells.
Growth factor ligands are produced, to which cells can respond via the expression of cognate receptors. Alternatively, cancer cells send signals to stimulate normal cells to support tumor formation reciprocating with growth factors. Somatic mutations were revealed after performed DNA sequencing analyses of cancer cell. Additionally, cancer cells can break away from this original mass, travel through the blood and lymph systems, and lodge in other organs where they can again repeat the uncontrolled growth cycle. This process of cancer cells leaving an area and growing in another body area is termed metastatic spread. All these hallmark process is summarized in the Figure 3.
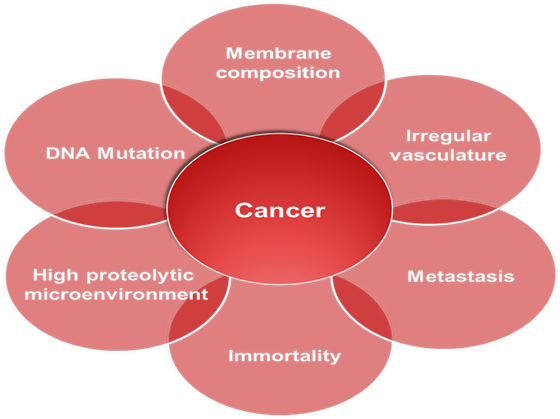
Figure 3 Hallmarks of Cancer. Different hallmarks are necessary for cancer progression. These hallmarks are characterized by changes in the nuclei eg, DNA damage; on the cell surface eg, the membrane composition, and its microenvironment due to the irregular vasculature, high protease concentration and finally the metastatic spread.
Over the last two decades, researchers have been trying to understand how cancer cells avoid the apoptotic machinery. The concept that programmed cell death, especially intrinsic pathway, by apoptosis serves as a natural barrier to cancer development has been recently established after compelling functional studies.20 The strategies of cancer cells to inhibit apoptosis are the deactivation of tumor suppressor function of P53, increase the production of anti-apoptotic proteins such as BCL-2, BCL-XL and diminish or inactivate the pro-apoptotic proteins including BIM, BID, BAD, BAX and BAK. The overexpression of anti-apoptotic proteins such as BCL-2, BCL-XL, and MCL-1, which bind to the BH3 α-helical domain of pro-apoptotic proteins such as BAX, BAK, BAD, and BIM, and inhibit their function.21
New therapies in Cancer based on the inhibition of pro-survival proteins
Recent studies are trying to develop new drugs using proteins that are very important to induce apoptosis. Pro-survival proteins (BCL-2, BCL-XL) contribute to oncogenesis and drug resistance inhibiting pro-apoptotic activity of BAX, BAK, BAD and BIM. BOD proteins such as BIM, BID, BIK, NOXA and PUMA can engage pro-apoptotic and anti-apoptotic proteins in the same way. Some researchers in the last decade have been working on discovering and construct possible molecules or peptides that could promote the activation of apoptosis (Figure 4).
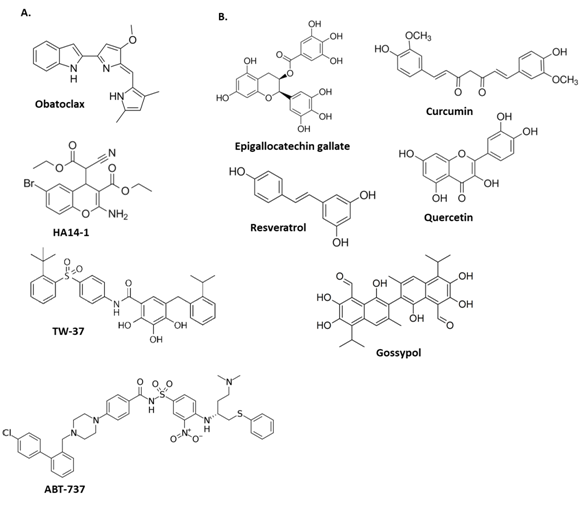
Figure 4 Chemical structures of some A. synthetic and B. natural small-molecule inhibitors of survival proteins BCL-2 and BCL-XL promoting initiation of apoptosis.
A lot of studies are focused on activation of BHD proteins (BAX, BAK). Letai et al.,16 found that short peptides representing the α-helical BH3 domains of BID or BIM are capable of inducing oligomerization of BAK and BAX releasing cytochrome c. BIM peptide is the BH3 domain of this protein that binds with BAX protein and it has been demonstrated6,22,23 that the sequence between residues 146-166 are sufficient to interact with BAX and activate apoptosis. Recent studies24 revealed that BH3 domain peptides (BIM or BID) with enhanced α-helical structures bound with greater affinity to BAX, activating cell death. The use of peptides as therapeutics is, however, limited by their low bioavailability, their inefficiency in crossing cell membranes (due primarily to their size), and their poor metabolic stability in vivo.25 To improve the poor physicochemical properties of peptides, Gavathiotis et al.,6 developed a stapling method to stabilized alpha-helix of BOD domains peptides (BIM SAHBs) that directly initiates BAX-mediated mitochondrial apoptosis. Aileron Therapeutics is a biopharmaceutical company that was founded in 2005 by Dr. Walensky,26 leading in the development of a completely new therapeutic modality that has an optimized version of the published Stapled BIM peptide into late-stage preclinical studies (Drugs.com). Other researchers are using this pathway to design inhibitors for pro-survival proteins as novel anti-cancer drugs. Most recent discoveries are using non-peptidic molecules (Figure 4A) that mimic the BH3 binding groove of pro-survival proteins disrupting BCL-2 or BCL-XL and MCL-1 binding to BAX, BAK, BAD, or BIM, freeing up pro-apoptotic proteins, which leads to the release of cytochrome c, activation of caspases and induction of apoptosis in a BAX- and BIM-dependent manner in human cancer cells.21 Other experiments reveal the benefits of natural polyphenols (Figure 4B) demonstrating remarkably broad therapeutic properties.18 These compounds are commonly listed as small-molecule Bcl-2 inhibitors and inhibits epidermal growth factor receptor, and metastasis-associated laminin receptor inducing apoptosis in tumor cells in vitro and in vivo.17 These results show different ways to activate intrinsic apoptosis directly or indirectly in cancer cells giving us multiple alternatives to design new treatments without side effects and low toxicity.
Concluding remarks
All the experimental apoptotic activators discussed in this review have biochemical mechanistic differences because they are trying to activate apoptosis interacting with BHD such as BAX or inhibiting BCL-2 but the similarity is that all utilize mitochondrial permeabilization pathway. Mitochondrion has a key role in a lot of processes that occurs inside the cell that allows sustainability, growth, or death; and in tumor growth could be similar. Cancer development could be stopped and the cell reprogrammed to kill tumor. The experiments performed to prove the effectiveness of these small drugs or peptides have great advantages above the actual approved clinical treatments such as chemotherapy and radiotherapy. Some of these new discoveries are on pre-clinical stages giving a hope to find better targeted treatments against cancer. But there are many different types of cancer affecting humans, including that each cancer could be developing in a different way depending on the organ or tissue. Therefore, these types of possible treatments cannot attack all cancer. Finally, these recent studies show that we are on the right way to understand the mechanism of cancer’s development. But obviously, a lot of studies have to be done first because the internal and external environment of the cell is a very complex place.
This publication was made possible by the support of the San Juan Bautista Research Center of the San Juan Bautista School of Medicine.

©2019 Delgado, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.