Journal of
eISSN: 2373-633X


Case Report Volume 8 Issue 4
Radiology Departement, Christus Muguerza High Specialty Hospital, Monterrey University, Mexico
Correspondence: Cuituny Romero AK, Radiologist, Radiology department of Christus Muguerza High Specialty Hospital and Monterrey University, Hidalgo 2525 Monterrey, Nuevo Leon, Mexico, Tel 8116117196
Received: July 27, 2017 | Published: August 30, 2017
Citation: Cuituny RAK, Onofre CJJ, Putz BMD, et al. An unusual case of ductal carcinoma in situ. J Cancer Prev Curr Res. 2017;8(4):311-315. DOI: 10.15406/jcpcr.2017.08.00285
Ductal carcinoma in situ (DCIS; intraductal carcinoma) is a noninvasive breast cancer originating from the cells that line the mammary ducts. Patients with DCIS can be asymptomatic at the time of presentation (radiographic findings on mammogram) or present with symptoms such as a palpable mass or nipple discharge.1 We present an unusual case of DCIS in a 40 year old woman with recent palpable mass. After diagnostic mammography and breast ultrasound, BIRADS 4c category was assigned and biopsy was performed. Pathology report indicates the presence of two histollogical types: DCIS and also invasive lobulillar carcinoma.
DCIS, ductal carcinoma in situ; CC, craniocaudal; MLO, mediolateral oblique; US, ultrasound; DLU, terminal ductal lobular unit; ADH, atypical ductal hiperplasia; LCIS, lobular carcinoma in situ
Ductal carcinoma in situ is defined by the proliferation of tumor cells within the terminal lobular unit with preservation of the basement membrane.2 Before screening mammography, DCIS accounted only the 0.8 to 5% of all breast cancers.2 Currently it represents 25% of all cancers in the US.2 It was first recognized in the early 20th century, mainly in mammography specimens with invasive malignancy.2 Studies indicate that it is a precursor of invasive carcinoma in an average time of 5 to 8 years.2 25-50% of women who have untreated DCIS will develop invasive cancer in the same quadrant of the breast. If ductal carcinoma in situ is not completely resected, 33% will recur and 50% of these will be invasive carcinoma.2 The American College of Radiology recommends annual screening mammography for women starting at age 40. Breast cancer incidence increases substantially around age 40 and even earlier for high risk women and women of color.3
40 years old female with an antecedent of cosmetic surgery with placement of breast implants 10 years ago. Her current condition is recent palpable area on right breast. Mammongraphy and ultrasound were performed on January 8, 2015 (Figure 1-10).
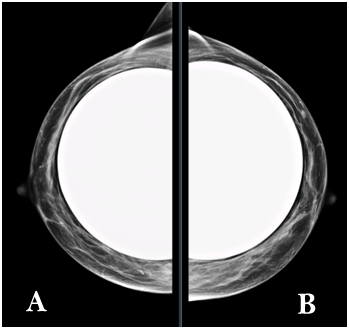
Figure 1 a) Right breast. b) Left breast. CC mammography views shows moderately dense fibroglandular parenchyma (pattern b ACR).
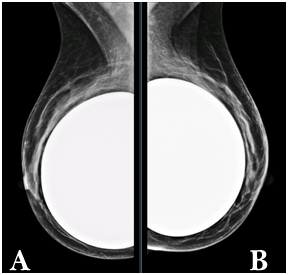
Figure 2 a) Right breast. b) Left breast. MLO views shows moderately dense fibroglandular parenchyma (pattern b ACR) with predominance in the upper quadrants. Retropectoral breast implants with preserved morphology.
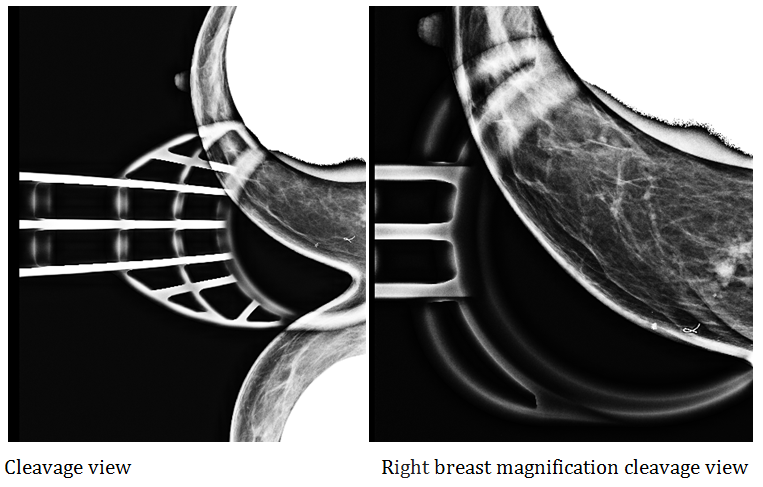
Figure 5 & 6 Rigth breast special views. In the internal upper quadrant of the right breast, there is an asymmetry located at 10cm from the nipple, evident in special views. It is nodular, irregular with spiculate and dense contours, occupying an area of 1.8cm. It is associated with distortion and some punctiform calcifications. Adjacent tissue shows a diffuse increase in density (suggestive of interstitial edema). These findings represent the palpable area.
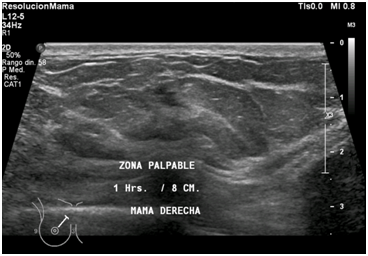
Figure 7 Right breast ultrasound was performed observing in the upper internal quadrant at 1 o´clock/8cm from the nipple, an irregular solid nodule with spiculated and poorly circumscribed margins. Adjacent tissue shows diffuse increase in echogenicity (suggestive of interstitial edema). These findings represent the palpable area.
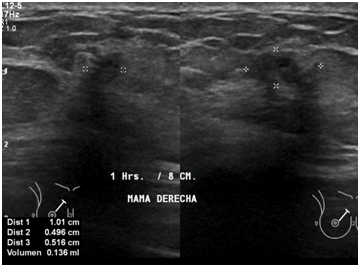
Figure 8 Right breast ultrasound show irregular nodule that measures 1.0 x 0.5 x 0.5cm, with heterogeneous echotexture and moderately hypoechoic. It is associated with calcification and echogenic halo.
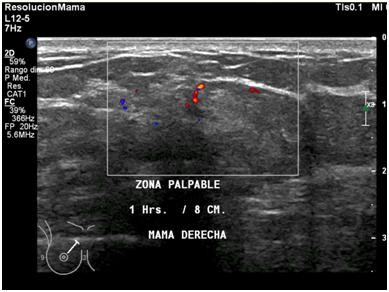
Figure 9 Right breast ultrasound with color doppler shows the presence of central and peripheral vascularity into the nodule.
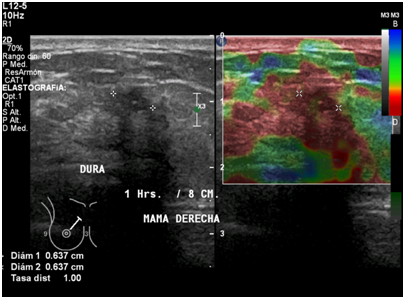
Figure 10 Right breast ultrasound with elastography shows hard consistency into the nodule, including areas of adjacent tissue.
Left breast without findings of interest on mammography and ultrasound. The palpable finding of right breast has an indeterminate appearance. Classifying BI-RADS category 4c: finding with high suspicion of malignancy. After this diagnosis, US guided needle biopsy of right mammary gland nodule showed intermediate grade in situ carcinoma with comedonecrosis. Immunohistochemistry demonstrated positivity for P63 and CD10 in the myothelial cells surrounding ducts showing carcinoma in situ (Figure 11-15).
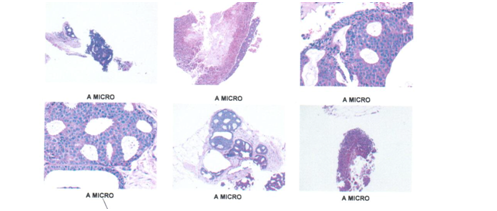
Figure 11 Fragments of right mammary gland were observed with few glandular units alternating with broad ducts showing an intermediate grade carcinoma in situ with cells that completely fill the lights and show their characteristic arches, the nuclei are polymorphic, molded and hyperchromatic and with abnormal mitosis. Tubeles with comedogenic pattern.
Limits of surgical section.
Internal (inked in blue) positive for infiltrant lobular carcinoma.
Superior internal angle (in-yellow painting) positive for carcinoma in situ.
Lower angle (inked in red) free of injury.
Carcinoma in situ. Internal limit (into in green) with lesion carcinoma in situ at 0.2 cms from the ink.
Surgical deep limit (inked in black at 0.5 cms of injury (carcinoma in situ)
The ductal system begins at the nipple. Between 25-30 galactophore ducts extend from the nipple and branch into segmental, subsegmental and terminal ducts. The terminal ducts are associated with lobules and form the terminal lobular ductal unit. In the normal breast, the terminal ducts and lobes are lined with a layer of epithelium and an outer layer of myoepithelium. Most breast malignancies arise within the TDLU.4
When the TDLU epithelium proliferates and there is more than one layer of cells it is called atypical ductal hyperplasia. If multiple cell lines proliferate or are cloned asymmetrically, the terminal duct or the lobule is filled with a heterogeneous population of small cells. This is called typical or benign hyperplasia. If the proliferating cells are uniform in appearance, the lesion is classified as atypical cell hyperplasia or DCIS grade 1 (low grade). The tumor is labeled as ADH if the abnormal proliferation is very small involving no more than two TDLU´s or 2 mm. If the tumor exceeds this size, it is labeled as DCIS.4
Although the finding of clustered calcifications on mammography is extremely sensitive for the diagnosis of DCIS, the specificity of this finding is only 10% to 35%, as a result of this relatively low specificity about 60-80% of biopsies by calcifications is associated with benign histological findings.4 DCIS and contralateral malignancy: 2.2 to 22% (DCIS or invasive cancer).4 Multicentricity in 12-80%. Defined by the presence of tumor foci in non-contiguous quadrants or at least 5cm.4 Multifocality is defined as the presence of more than one lesion in a single quadrant or within a radius of 5cm.4 Hollland and colleagues, demonstrated that DCIS spreads contiguously in the same ductal or adjacent system.4
Studies have investigated the utility of pathologic grading of DCIS for predicting the risk of recurrence after conservation therapy. The simplest, most reproducible classification system is the Van Nuys system, which identifies three groups of DCIS lesions. In the Van Nuys system, lesions are differentiated first according to nuclear grade (high grade or other grade) and then according to whether necrosis is present or absent.5 The Van Nuys Prognostic Index, adopted from a review in which a risk category was developed based on margin status, histologic subtype, tumor size, and patient age using a cohort of DCIS patients treated from 2 institutions, continues to be used by some practitioners as part of their decision-making process for adjuvant radiation after local excision. It is important to note that the data from this “scoring system” were derived from retrospective data and that all randomized prospective data published to date have consistently demonstrated an improvement in local control in all patients.6
Incidence of comedonic type (aggressive) has been maintained. Incidence of noncomedonic has increased from 15 to 22 times. 68 to 92% of cases are detected as calcifications on mammography. 2-3% as an asymmetry.2 Immunohistochemistry (IHC) is used to characterize intracellular proteins or various cell surfaces in all tissues. Individual markers or more often panels of various marker proteins can be used to characterize various tumour subtypes, confirm tissue of origin, distinguish metastatic from primary tumour and provide additional information which may be important for prognosis, predicting response to therapy or evaluating residual tumour post-treatment.7
The most important diagnostic problems that occur in mammary gland tumor pathology are: the differential diagnosis of various types of benign lesions and carcinoma; differentiating between carcinoma in situ and invasive carcinoma, diagnosis and differentiation of microinvasion and its imitating lesions and confirming the breast as the primary site in metastatic carcinoma.7 p63 is a homolog of p53, it has now been shown that about 10% to 15% of invasive tumors, particularly high-grade and metaplastic carcinomas, express p63, although the staining is usually weaker than that seen in myoepithelial cells. Similarly, foci of squamous differentiation stain positively.7
CD10-positive cells have been reported in the stroma of prostate, breast, colorectal, and lung carcinomas.8 CD10-cell surface zinc-dependent metalloproteinase, has been demonstrated on the stromal cells of some breast carcinomas, and suggested to be upregulated in breast cancer cells. Some experimental data indicate that CD10 may be a potential target for new cancer therapies, as it is involved in cleavage of doxorubicin, critical component of many cancer treatment protocols, and results in chemoresistance. Inhibition of CD10 enzymatic activity may enhance the antitumor efficacy of traditional chemotherapeutic regimens.8
This case illustrates the importance of performing mammography in women at age of 40. In this particular patient, we have the antecedent of breast augmentation 10 years ago with no apparent control with some imaging method. If the patient also has symptoms or palpable mass, the study we will perform will be diagnostic as the patient will come for a specific problem and we will have to perform the work-up with additional mammography, ultrasound or breast MRI if necessary, in order to integrate the information and give a BIRADS category and take the appropiate measures.
This patient returned to the breast imaging deparment for a preoperative marking of the lesion in the right breast and we had the opportunity to see the postoperative pieces by image that allowed us to corroborate the findings identified in the diagnostic studies. Finally, to know that the patient not only had carcinoma in situ but also inasive lobular carcinoma which allows the clinician a more optimal approach for the appropiate management and treatment of the patient. Patients need to be informed that routine mammography should be performed at age 40 even if there are no symptoms. If the patients have a significant family, personal or surgical history, the control with imaging methods should be performed before age 40, either with mammography or ultrasonography depending on the personal clinical history in each patient, the best method for monitoring will be suggested.
American collegue of radiology (ACR) reccomends to woman with breast augmentation:9
None.
Authors declare there are no conflicts of interest.

©2017 Cuituny, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.