Journal of
eISSN: 2373-633X


Case Report Volume 9 Issue 5
1Assistant professor, Department of Radiation oncology, Cancer Institute (WIA), India
2Professor/HOD, Department of Radiation oncology, Cancer Institute (WIA), India
Correspondence: Harish kumar K, Assistant professor, Department of Radiation oncology, Cancer Institute (WIA), Adyar, Chennai-20, India
Received: June 01, 2017 | Published: September 10, 2018
Citation: Kumar H. Merkel cell carcinoma of cheek– response to radiation – a rare case scenario. J Cancer Prev Curr Res. 2018;9(5):222-224. DOI: 10.15406/jcpcr.2018.09.00355
Merkel cell carcinoma is a very rare cutaneous tumour, which is aggressive and related to high mortality. We are presenting a 50yrs old female patient reported which large ulcer involving right side of face which is painless and associated with right side cervical nodes. Biopsy from ulcer revealed Merkel cell carcinoma. She received concurrent chemoradiation with Etoposide/Cisplatin. Chemoradiation was chosen as first line therapy as it was deemed inoperable. A complete objective response was achieved at end of therapy.
Keywords: merkel cell carcinoma, external beam radiation
Merkel cell carcinoma (MCC) is a rare, aggressive, neuroendocrine carcinoma of the skin that originates from Merkel cells of the dermoepidermal junctions, although some recent work proposes pluripotent dermal stem cells to be origin of this neoplasm.1 The annual incidence is 0.6 per 100,000 persons2 but is apparently increasing in the last years thanks to more accurate diagnostic pathology techniques, an aging population, increased sun exposure, and improved registry tools. MCC has a high mortality rate, the overall 5-year survival rates ranging from 30% to 64%.3 Males are more often affected than females, the median age at diagnosis being 70years.2 It is extremely rare in children, with only a few cases reported in literature.
Ultraviolet radiation exposure, chronic immune suppression (especially from chronic lymphocytic leukemia, human immunodeficiency virus, and prior solid organ transplant) and the Merkel cell polyomavirus are the main risk factors involved in the tumour pathogenesis.4 Concerning the latter, many reports described a strong correlation between infection and carcinogenesis, although the presence of the virus itself is not sufficient to induce MCC.
Clinically, the lesion appears as a fast-growing, painless, solitary dermal nodule, firm, non-tender, coloured from red to violet; usually present as an ulceration. Skin of the face, arms and lower limbs are the most common sites of localization whereas the trunk and oral and genital mucosa are rare.2 Typical clinical features are summarized in the acronym “AEIOU” proposed by Heath et al.5 Asymptomatic, expanding rapidly, immunosuppression, older than age 50 and ultraviolet-exposed site.
The approach to disease management includes with a complete physical examination followed by imaging. Treatment strategies are best considered in a multidisciplinary board consultation. The surgical approach, when negative margins are possible and the disease is not disseminated, should be the first choice followed, when the risk assessment contemplates it or specific lymph nodes are involved, by adjuvant radiation therapy within 4weeks. Adjuvant chemotherapy in regional disease could be considered depending on clinical judgment. In cases of disseminated disease, chemotherapy represents first line therapy; the choice of the agents to be taken based on clinical judgment and experience.
Here we present a case of MCC in a middle aged female.
An 50-year-old female presented with a pedunculated ulcerated mass with fumigations mainly in the right cheek, extending subcutaneously into to the pre maxillary region, pre auricular and parotid area measuring 5x5cm (Figure 1A) (Figure 1B). There was no intra oral lesion suggesting its pure dermal lesion clinically (Figure 2). There was large matted nodal mass clinically involving sub mental, right Ib/II/III/IV and V measuring 8x7cm, hard fixed mass. The lesion had been enlarging for more than three months. The patient had no history or evidence of co morbidities; punch biopsy was performed and reported as Merkel cell carcinoma after IHC co relation.
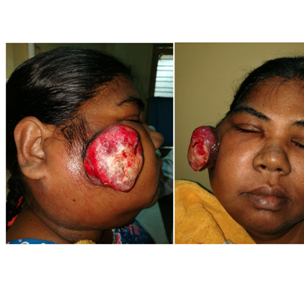
Figure 1
The immune histochemical phenotype of the dermal-located malignant cells was characterized by dot-like focal positivity for Cytokeratin 20 (CK20+), diffuse positivity for synaptophysin(+), cytokeratin AE1/AE3, CD99+, and strong nuclear positivity for Ki-67 (+100%). There was negative staining for chromogranin, CEA-TTF1-, CD56-, S100-, CD20-, CD79a-, CD3-, CD23-, CD5-, CD10-, and Cyclin D1.
PET CT scan showed exophytic cutaneous soft tisse mass seen in right premaxillary region of size 4.5x4.6x4.9cm (SUV–26.5), Conglomerate matted nodal mass seen in right neck involving submental, submandibular, interparotid, parapharyngeal , level II, III and IV–9.0x5.1x12.1cm infiltrating IJV and displacing carotid without infiltration–(SUV–18.4) and discrete neck nodes involving submental region (SUV 21.3) and physiological bone marrow uptake (Figure 4A–4D).
The tumour was classified Non Metastatic Merkel cell carcinoma of Right side cheekcT4N3M0, locally advanced. Based on these clinico–radiological findings, the history, and on her age, she was planned for definitive chemo radiation after multi disciplinary board discussion

Figure 3
Planning CT Scan was done with proper immobilisation using thermoplastic mould. 2mm cuts axial CT scan was taken from vertex till carina level. Images were transferred to Varian planning system and image registration done with PET CT images. GTV was regenerated by contouring primary tumour and neck nodal disease. CTV was generated by 2cm margin and ipsilateral whole neck in view of N3 disease status. PTV was generated by 1cm margin to CTV. Using Rapid Arc therapy with SIB technique, she received 66Gy RT to high risk PTV which includes primary and nodal disease and 60 Gy RT to intermediate risk PTV over a period of 6weeks along with concurrent Etoposide/Cisplatin chemotherapy–(Figure 5A) (Figure 5B).
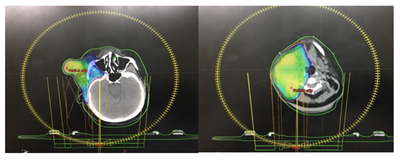
Figure 5
Patient had more than 60% response clinically at end of 4 weeks, hence underwent adaptive RT planning at 40Gy. She developed grade 4 afebrile neutropenia requiring Inj GCSF for 3days and Gr 2mucositis as per RTOG criteria. RT was pended for 1week during morbidity. No other significant morbidity. Overall she tolerated treatment well
At end of treatment the tumour showed a significant regression of disease (Figure 3A) (Figure 3B). By 1 months there was evidence of complete objective response and she was planned for 2 more cycles of Adjuvant EP Chemotherapy
First described as trabecular carcinoma in 1972 by Toker,6 MCC represents an aggressive, primary cutaneous carcinoma incorporating both epithelial and neuroendocrine features. The diagnosis is made by clinical evaluation and biopsy, although other small round cell tumors may be considered. For this reason a complete immunohistochemistry panel is needed for the correct diagnosis. Cytokeratin 20 (CK-20), a marker of epithelial origin, is a very sensitive marker for MCC7 since it is positive in 89-100% of cases. Together with negativity of transcription factor 1 (TTF-1), it provides the greatest sensitivity and specificity to exclude small cell lung cancer,8 although N-specific enolase, synaptophysin, and chromogranin-A represent markers of neuroendocrine origin with possible positivity. In our case, pathology and immune-histochemical markers, along with clinical features confirmed the diagnosis.
MCC generally shows a malignant behaviour, with regional lymph-nodes as well as distant metastasis being frequently involved. Twenty-five percent of patients present with lymphadenopathy and 5% with a distant metastasis. Skin, lung, nervous system, bone, and liver are the most frequent secondary locations.9 For this reason lymph-nodal examination should be performed. Additionally, PET/CT is often useful for complete staging.
Surgery plays the key role for clinically localized MCC and a complete surgical excision with 2cm safety margin, if feasible, seems to be the best treatment approach. Adjuvant radiation with 50Gy to the tumour bed and regional lymph-nodes is also recommended, especially for advanced local and regional disease.10 When surgery is not feasible, radiation therapy alone, or combined with alternative therapy (e.g., chemotherapy) should be considered.
Presently, there is no first-line chemotherapy established for MCC, since no controlled randomized trials exist. Only retrospective case series and case reports are available. The chemotherapy regimens used have combined carboplatin or cisplatin with etoposide, cyclophosphamide with vincristine, doxorubicin, bleomycin, or 5-fluorouracil.
Despite a good initial response, early recurrences are the rule. In a retrospective analysis including a wide number of patients, adjuvant chemotherapy was linked to a worse overall survival compared to patients who did not received chemotherapy.3
Concurrent chemoradiation is wiser option for locally advanced inoperable tumour, which helps in acceptable local control and considerable down staging. Tumour response can be assessed at end of pre op EBRT dose for feasibility of operability. If still inoperable then considered for definitive chemoradiation followed by adjuvant chemotherapy.
In our patient, considering large volume locally advanced – deemed inoperable disease. Patient was taken up for definative chemoradiation followed by adjuvant chemotherapy with EP – total 4 cycles. Our patient achieved a complete objective response in a short period of time. However, long term follow-up is needed to rule out possible recurrence.
None.
Author declares that there is no conflict of interest.

©2018 Kumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.