Journal of
eISSN: 2373-633X


In the evolution of metastatic prostate cancer, the most frequent metastatic involvement is bone, followed by lymph nodes out from pelvis, and less frequently the visceral involvement. The nervous system spread is very rare and late, and extraordinarily rare leptomeningeal lesions, finding few references in the literature. The clinical features are characterized by neurological deficit at different levels and fast deterioration.
We describe the case of a patient 77 years old, affected by very high-risk prostate cancer in initial stage T3bN1M0 treated with Radiotherapy the pelvic volume, seminal vesicles and prostate, combined with androgen deprivation for 24 months. At 32 months follow-up, the patient presents fast neurological loss and radiological diagnosis of metastatic leptomeningeal involvement above lumbar and sacral levels. We proceeded to urgent irradiation of those affected spinal segments, with good initial response and complete neurological recovery. Later, the patient presented again fast and progressive deterioration, with multiple organ failure that caused his death.
Keywords: prostate cancer, leptomeningeal involvement, extra-medullary, intra-medullary
Prostate cancer is the first solid malignancy in men, and second death cause due to cancer in this group.1 Until few years ago, patients with locally advanced tumor were treated with surgery, androgenic deprivation or radiotherapy, with poor and non-homogenous outcomes. Most patients died with metastatic bone disease, node disease or, less frequently, visceral metastases. Brain involvement was not relevant2 and leptomeningeal affectation was extremely rare.3 Intra-Dural extra-medullar affectation is unusual in any cancer, about 5%4 and referred to prostate cancer is exceptional, so very few cases were reported to literature in the last years. This fact drives to the supposition that there could be a factor which modifies classical evolution of prostate cancer and, as many other malignancies, the introduction of new therapies such as hormone therapy and chemotherapy in association with those previously established.
77 years old man patient comes to Emergency Services afflicted by functional impotence of both legs since a few days ago, without pain associated.
Clinical story: In 2009, was diagnosed of prostate cancer with PSA 6.82 ng/mL, Gleason 6, and clinical CT staging T3bN1M0 by evolvement of seminal vesicle and an iliac node. Treated with External Beam 3D-Conformed Irradiation over pelvis, seminal vesicles and prostate plus androgenic deprivation for 24 months. After 30 months of follow-up, PSA is elevated (10 ng/mL) with asymptomatic patient. A new thoracic-abdominal-pelvic CT study is performed, as bone-scan, which shows osteoblastic metastatic bone disease in sacral location and left sacral-iliac joint (Figure 1). Hormone treatment is reintroduced with complete androgenic deprivation, achieving an auspicious biological response with the descent of PSA quantification (3.83 ng/mL).
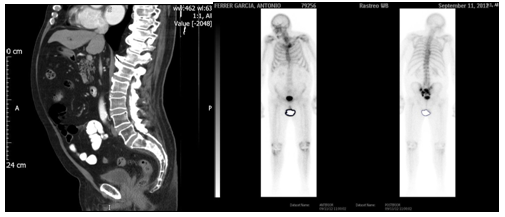
Figure 1 CT scan shows Osteoblastic metastatic bone disease in sacral location and left sacral-iliac joint.
6 months after beginning hormone therapy, this patient took advice of one-month evolution clinical progressive pain in sacral bone and left sacral-iliac joint referred to both legs. PSA elevation to 21.76 ng/mL is noticed with testosterone in castration levels. A new Radiotherapy treatment was given (30 Gy in 10 sessions of 3 Gy) as painkiller purpose in sacral and left sacral-iliac bone disease. At follow-up, this patient stayed asymptomatic and carried an active life, with stability of disease.
5 months later, the patient came to Emergency Services with short-time evolution of functional impotence and strength loss in both legs. Neurologic exploration presented the next situation: higher neurological functions and cranial nerves inside normality, arms without deficit, strength loss 2/5 in left leg and 3/5 in right leg, preserved sensitivity, tendon reflexes preserved, and plantar reflex in flection. No pain touching or precutting sacrum, pelvis or lumbar spine. Neural-axis MRI study revealed bone metastasis in sacrum, already known, and leptomeningeal spread with tumor lesions dependent of intra-dural and outside spinal cord meningeal with some other lesions inside and outside spinal cord at thoracic level (Figures 2 & 3). In dural bag, nodular injury follows radicular (Figure 4). No new diagnostic tests were performed because of progressive disease nature.
Those findings led to one more Emergency Radiotherapy treatment, set on by 30Gy in 10 sessions of 3 Gy over both thoracic and lumbar locations. Treatment first response was positive, with partial strength recovery of legs and the beginning of functional rehab program.
One month after the last active treatment, the patient entered in hospital again with progressive general status fail, dark urines, dyspnea, yellow spit and later clinical delirium, complications that led him to death.
Leptomeningeal spread of cancer is an event few times diagnosed, so the frequency of this found is only 5% in solid malignancy autopsy series, and mostly from lung or breast cancer. Thus, urologic tumor origin is rare, and exceptional in prostate cancer,5 less than 1%.6 In our case, first disease manifestation is a biochemical relapse with asymptomatic patient, and CT and bone-scan showing sacral and left sacral-iliac osteoblastic affectation. Later, sacral-iliac pain appears, so this symptom with the complementary studies lead on to conclude that bone metastatic disease is our indication to deliver palliative treatment, with positive initial outcome and pain disappearance. This evolution, usual in palliative prostate cancer treatment, supports the common decision of not performing new complementary tests such as neural-axis MRI.
Besides, analyzing the feasible reason, which has generated leptomeningeal invasion in our patient and revised literature about this clinic presentation, we think the mechanism could have been double. In the one hand, leptomeningeal spread could have happened by closeness to bone metastases in sacral spine, which invaded spinal column and cord. In the other hand, peripheral neural invasion through spinal nerves could be a reason. Hentschel et al.7 describe a case of intra-dural spine cord and extra-spine cord affectation and, as in our case, previous multiple bone disease was confirmed, so it was impossible to spare neurologic affectation due to leptomeningeal metastases from a first symptom like back pain preceding neurologic failure.
Our patient presents at MRI nodular lesions, which follow emergent nerves and could have been the first event that had developed neuropathic component in left leg. So, Demetriades et al.8 present a case of metastatic prostate cancer which first spine cord involvement manifestation happens with no bone disease, holding the hypothesis that prostate cancer could progress through spine nerves and eventually reach out arachnoid space. Evenly, Hérbert-Blouin et al.9 ensure in their work that leptomeningeal involvement in prostate cancer through lumbar-sacral plexus is not so unusual when high resolution MRI studies are performed, so they suggest it should be noticed when multiple neurologic deficits.
According to long time elapsed from diagnose to first neurologic radiculopathy symptoms appearance, and later aggressive evolution with fast deterioration of the patient, we think that the presence of low spine involvement as first metastatic disease in prostate cancer makes necessary complementary tests in order to search intentionally the feasible peripheral neural spread to spine cord roots such as is told in the publication of Hérbert-Blouin et al.9 where image findings are often confusing and, thus, intra-neural tumor spread till lumbar-sacral plexus could be more frequent than we have thought until now.
High-resolution MRI and PET/CT studies support the hypothesis of direct perineural propagation of prostate cancer through pelvic plexus to lumbar-sacral plexus and this mechanism would also explain the leptomeningeal metastases in this cancer.
Continuing with our case analysis, in MRI thoracic cord lesions are described as suggestive of intra-spinal location (Figure 4). This fact seems very relevant according to two aspects. Firstly, in reviewed literature are described possible mechanisms of invasion that are present in solid tumors at higher levels in leptomeningeal location when beginning is sacrum, or blood spread or direct involvement of intra-dural extra-cord component5,10 which would explain our case. And secondly, this figure is not described in the reviewed literature in any prostate cancer, so we consider that is a singular fact different from the published ones before and, in our opinion, could be one step closer to progression of disease.
Leptomeningeal spread of tumor through CSF comes supported by many studies, which confirm until 80% coincidence of brain metastases with intra-dural and extra-spinal metastases by leptomeningeal spread.4,11 In our case we do not think it happened that way, because there was not cognitive deterioration, intracranial hypertension symptoms or cranial nerves alteration in neurological exploration at diagnose, and it seems alike that initial spread was through lumbar-sacral plexus with later involvement of higher levels.
Fast lethal progression in our patient coincide with that referred in existing literature evenly, and thus we are in front of a very aggressive process in prostate cancer that, even if it is rare, it shall be thought about when miscellaneous neurologic deficit patient is found.12,19
None.
None.
The authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.
 Month of October is here, which is commonly known as the World Breast Cancer Awareness Month, The major aim of this program is to Educate people on multiple ways on the prevention and early detection of breast cancer. As part of this, we are doing our best to spread its cognizance by accepting articles on this topic and grab best discount of 30% for your submissions to our Journal of Cancer Prevention & Current Research (JCPCR).
Month of October is here, which is commonly known as the World Breast Cancer Awareness Month, The major aim of this program is to Educate people on multiple ways on the prevention and early detection of breast cancer. As part of this, we are doing our best to spread its cognizance by accepting articles on this topic and grab best discount of 30% for your submissions to our Journal of Cancer Prevention & Current Research (JCPCR).