Journal of
eISSN: 2373-633X


This study determined the impact of vitamin C dosage on genistein-induced apoptosis in LNCaP cancer cells in vitro at various treatment regimens. Although the linear regression of viability assay (MTT) indicated a p-value = 0.11; NBT assay reveal a declining SOD activity during cell death. Apoptosis induction was the main mode of treatment induced cell death. The overall data showed the trend of treatment efficacy as; (Gen 10uM + Vit C 40uM) > (Gen 30uM + Vit C 40uM) > (Gen 70uM + Vit C 40uM) >10uM genistein > 70uM genistein. The chi-square test for comparing necrosis, apoptosis, and live cells showed that Vitamin C could impact genistein-induced apoptosis in LNCaP cells (p = 0.0003). This study forms basis for in vivo studies of the impact of vitamin C on genistein-induced apoptosis in LNCaP prostate cancer cells.
Keywords: viability, apoptosis, necrosis, cell death, treatment, efficacy, in-vivo, induction, antioxidant, in-vitro
Vit C, Vitamin C; Gen, Genistein; NBT, Nitro Blue Tetrazolium, DMSO, Dimethyl Sulfoxide; MTP, Microtiter Plates; ROS, Reactive Oxygen Species
Prostate cancer
In 2012, the most commonly diagnosed type of cancer among men was prostate cancer (PCa) accounting for 29% of all new cancer cases. In 2012, PCa ranks second to lung cancer in cancer-related deaths and these accounts for 9% of cancer death in men.1 Despite the large investments in cancer research and the wealth of knowledge and discoveries in cancer biology and genetics, prostate cancer is still at best, minimally controlled by modern medicine.2,3 The mortality rate for cancer in the 21st century is the same as it was 50 years ago. Prostate cancer is the most common form of non-cutaneous cancer and the second cause of cancer-related deaths in American men, with 1 out of every 7 men projected to be diagnosed with prostate cancer during their lifetime. An estimated 220,800 incidences and 27,540 deaths occurred in the year 2015.4 Notable progress in cancer treatment has been achieved due to persistent pursuit of research in therapeutic regimens. These treatment regimens have allowed the locally confined disease to be treatable. However, the metastasized prostate cancer still poses therapeutic challenges. Consequently, prognosis still remains unclear. In addition to surgery, standard chemotherapy has been the most effective treatment regimen. However, such therapies have their limitations in terms of safety, acceptability, and adaptability, apparently due to incomplete elimination of the metastasized cancer and the serious side-effects.5 Introduction of alternative strategic therapeutic regimen has been the interest of research endeavors. Current approach in therapeutic research is focusing on attacking cancer cells at the molecular level in order to initiate signal transduction inhibitors, apoptosis-inducing molecules, and growth inhibitors since the 1990s.6 This means identifying specific cancer-associated biomarkers/proteins, growth promoting molecules, and signaling pathways within the cells that could be specific targets for therapeutic agents. DHA is taken up into cells by glucose transporters7,8 Inside the cell, it is reduced to ascorbic acid7,8 and reduces intracellular ROS levels, thus acting as an antioxidant.9–11 Ascorbate is considered to be an important antioxidant in extracellular fluid.12 It also guards against aqueous radicals in blood13 and protects plasma lipids from peroxidative damage caused by peroxyl radicals.14 Conversely, ascorbate also accelerates oxidative metabolism by preventing the use of pyruvate for glycolysis.15 This property helps to inhibit the proliferation of tumor cells, but not normal cells.16,17 In a great number of malignant cancer cell lines, it is quite interesting that the cytotoxic effect of ascorbate is correlated with its pro-oxidant activity.18–22 High-dose intravenous (IV) vitamin C has been administered by medical practitioners for cancer patients.23 Vitamin C administered intravenously omits the controlled intestinal absorption mechanism and results in significantly higher plasma concentrations than are obtained through oral intake.24 When the intake of vitamin C is below a critical amount (10 mg/d) for prolonged periods, the result often leads to; failure of wounds to heal, petechial hemorrhages and follicular hyperkeratosis. This leads to a condition known as scurvy.25 It has been proposed that at these high-doses vitamin C acts as a pro-drug via metal ion-dependent generation of cytotoxic hydrogen peroxide.26 However, other potential anti-cancer mechanisms, such as regulation of epigenetic marks and transcription factors, are also possible.27 The increased use of dietary supplements for prostate cancer is occurring despite data showing consumption of some supplements actively promoted for anti-prostate cancer activity actually increased the risk of prostate cancer.28 The role of ascorbate (vitamin C) in cancer treatment has been a topic of controversy.29,30 The core of this argument is the lack of reproducibility in regards to the therapeutic effects of ascorbate on cancer patients.31 This leads to a problem compounded by uncertainties associated with deficiencies of independent pathologic confirmation and failure to include appropriate placebo groups in clinical studies.32-35 On the basis of an initial study of the antitumor effects of ascorbate in 50 patients with advanced cancer, Cameron and colleagues concluded that high-dose ascorbate improved treatment outcome,36 showing that the long-term survival of cancer patients who received high-dose ascorbate supplements was 20 times greater than that of patient in the control group.37 However, Moertel et al.33 concluded that there was no significant difference in survival between ascorbate-treated and -untreated groups.33,34 Conner and colleagues reported that all antineoplastic drugs tests produced mitochondrial dysfunction, including loss of mitochondrial membrane potential and an increase in ROS levels (this phenomenon was inhibited by vitamin C). They postulated that vitamin C acts as an antioxidant to protect cells against mitochondrial dysfunction induced by antineoplastic agents, and thus antagonizes the cytotoxic effects of antineoplastic drugs.38 In a similar vein, Blair cautioned that because vitamin C/d (200 mg) induced decomposition of lipid hydro-peroxides to endogenous genotoxic, it might be counterproductive in cancer treatment.39,40 Patients undergoing chemotherapy commonly experience fatigue, with more than 75% of patients reporting feelings of debilitating tiredness or loss of energy.41 Fatigue sets in after chemotherapy and increases incrementally with consecutive cycles of chemotherapy.42 Fatigue may even persist for years after treatment completion.43 Regarding genistein single treatment, a study reported that genistein treatment impacted considerable side effects in almost 20% of participants.44 Although cancer-related pain can be managed with opioids,45 no effective therapy for fatigue has yet been identified. However, more recent studies on the therapeutic effects of vitamin C have provided a clearer understanding of its effect in cancer treatment. One hypothesis was that ascorbate exerts an antitumor effect by increasing collagen synthesis.46,47 High intake of ascorbate initiates a reduction in mitochondrial membrane potential and a liberation of cytochrome c from mitochondria to fluid component of cytoplasm (containing the insoluble, suspended cytoplasmic components), enhancing apoptosis. Low intake of ascorbate induced cell-cycle arrest of cancer cells.48,19 As a result, the effect of ascorbate on cancer cells was initiated by an increase in intracellular ROS levels. Levine and colleagues have also showed anticancer activities of ascorbate that were derivable to its pro-oxidant properties, showing that ascorbate acts as a pro-oxidant and decreases tumor growth in mice.50 They also indicated that ascorbate produced hydrogen peroxide-linked cytotoxicity in numerous cancer cells without affecting normal cells. More significantly, Levine proposed that ascorbate-initiated formation of hydrogen peroxide preferentially occurs in extracellular fluid compared with blood.51 These studies provide a mechanistic basis for applying ascorbate as a pro-oxidant therapeutic agent for cancer treatment. In this study, the additive effect of vitamin C on genistein chemo-Enhancement of Genistein-Induced Apoptosis in LNCaP Prostate Cancer cells preventive action was assessed using MTT assay and florescence assay. (The ratio of percentage apoptotic death to percentage necrotic death was determined for each treatment group). The action of ascorbate on cancer cells has also been more clearly defined by in vitro studies. This study summarizes the biological mechanism of action of ascorbate (adjuvant) in cancer therapy in addition to genistein (as a phytotherapy).
After the cells reached their log phase, trypan blue assay was carried out. Based on the result from the trypan blue assay, 1x104 cells were seeded in each well of the 96-well microtiter plate and the microtiter plate placed in the incubator. After 24 hours the cells adhere to the surface of the plates and are now at 80% confluence on the 4th day of cell culture. On the 5th day, the LNCaP cells were assayed using the NBT solution to access Superoxide Dismutase activities going on in the cells and MTT solution to access the anti-proliferative effect of the three treatment groups (Figure 1).

Figure 1 LNCaP cells were cultured in 25m2 culture flasks and placed in the incubator at 37°C and 5% CO2 to grow.
Drugs (Micronutrients) Genistein (4’, 5’ 7- trihydroisoflavone) was purchased. Genistein diluted into solution with DMSO (Dimethyl sulfoxide) to make an 80μM stock solution. Treatments in concentrations of 0, 10, 30, 40, 50 and 70 µM (G0-G70) were prepared.52,53 and stored at 3°C until used. Vitamin C supplement was dissolved in distilled water to make a 100μM stock solution. Aliquots in concentration of 0, 10, 30, 40, 50, 70 μM (VtC0-70) was stored at 3°C until it was needed. MTT assay, NBT assay, and Acridine orange/ Ethidium bromide (AO/EB). LNCaP prostate cancer cell lines were purchased from American Type Culture Collection. LNCaP cells were cultured in RPMI 1640 complete medium (ATCC, VA) and maintained as mono layers in 75cm2 tissue culture flasks (Sigma Scientific, St. Louis, MO, USA). LNCaP cells were plated in triplicates into 96-well microtiter plates (MTP) at a concentration of 1.0×104 cells per well. The plates were incubated in humidified CO2 incubator at 37°C and 5% CO2 for 48h to attain 80-90% confluency. The cells were then subjected to various treatments. The cells were treated with one of the following treatment regimens: varying concentrations of genistein (Gn0-70); varying concentrations of vitamin C (VtC0-70); combination of Gn and Vit C (Gn0-50 + VtC40). From our preliminary study, the IC50 (i.e. the concentration of Vit C at which 50% of the cells were killed or inhibited) was observed to be 40µM. In this study, Vitamin C at 40µM concentration (IC50) was used in the VtC-Gn combination treatment of LNCaP. The cells were then incubated for 24 to 48 h at 37°C and 5% CO2. In order to determine the anti-proliferative or growth inhibitory effects of genistein and/or vitamin C treatments on each prostate cancer cell line, MTT assay was utilized. The MTT dye detects metabolic activity through preferential conversion of viable cells into purple colored formazan.
The enzyme mitochondria dehydrogenase is only present in the effective power house of living cells, and is needed for cleaving the tetrazolium substrate into insoluble formazan. The color strength produced is directly proportional to the number of viable cells (metabolically active cells) and numerically calculated by optical density from individual wells. Briefly, LNCaP cells were plated onto 96 well plate each at a density of 1.0 × 104 cells per well. The cells were then incubated with graded doses of genistein (Gn0-70) for 24 h. In the combination treatment group, cells were treated with genistein (Gn0-70) and vitamin C (40 µM). 100 µL of MTT (2.5 mg/mL in PBS-phosphate buffered saline) (Sigma Scientific Chemical Co., St Louis, MO, USA) solution was poured into each well of the MTP and placed in the incubator for 4 h at 37°C and 5% CO2 to allow for the reduction of MTT into formazan by metabolically active cells. The undissolved formazan particles formed were solubilized or lysed to release the formazan product by adding 100μL of disintegrating solution (DMSO) to pellets formed. LNCaP Cells from each well were harvested (by vigorous aspiration), poured to a labeled small centrifuge tube and centrifuged for 10 minutes at 5000RPM. Any unabsorbed MTT dye was eliminated by decanting the supernatant. The LNCaP cells in each tube were transferred into different wells of a 96-well MTP. The Elisa plate reader measured values at 490nm with an automated micro plate reader (Biotech, Vermont, USA). Live cells were determined numerically based on the optical absorbance (optical density, OD) of the sample. The mean percentage of post treatment viable cells relative to the controls was calculated as shown earlier.
Nitro blue tetrazolium assay
Cells have numerous defense mechanisms against impact of reactive oxygen species (ROS). In this assay, superoxide ions (O2 -) generated by graded genistein and/ or vitamin C treatments converted NBT to NBT diformazan. SOD decreases the O2-concentration and thereby lowers the rate of NBT-diformazan formation. LNCaP cells (with Gn and /or Vit C) were subjected to NBT assay. The extent of treatment-induced decrease in the formation of NBT diformazan is an estimation of SOD activity present in the cancer cells. As the absorbance increases the amount of superoxide anion (ROS) formed, also, increases. The SOD enzyme as an inhibition activity can be estimated by calculating the reduction in the absorbance at 490nm, whereas an increase in absorbance shows treatment-induced increase of intracellular ROS liberated by LNCaP cancer cells as shown by Oseni et al.51 Briefly, 40μL NBT solution was poured into the adherent cells in each well of the corresponding MTP, and placed in the incubator for 4 hours at 37°C and 5%CO2. The cells were dissociated with trypsin, pipetted into individual micro-centrifuge tubes, and equal volumes of PBS were used to dilute it. Centrifugation of the cell suspensions was done and pellets collected. The pellets were rinsed four times with DMSO to break down the cell membranes. The clear liquid overlying the solution were then pooled for absorbance reading on micro plate reader to estimate the ROS liberation levels. The ROS levels were expressed as percentage of ROS (or SOD inhibition) relative to the control. The means of the absorbance were graphed against the mean concentrations of the drugs/reagents.
Fluorescence microscopy
Assay is performed for differential cell death detection purpose. Acridine orange and Ethidium bromide dyes possess dissimilar fluorescence emission spectra. Therefore a cocktail solution of the two markers can be used to make a distinction between viable, apoptotic, and necrotic cells. Acridine orange permeates both viable and non-viable LNCaP cells, stimulating the nuclei to radiate green fluorescence. Ethidium bromide permeates dead cells (with compromised cell membrane integrity) and selectively stains the nuclei (nonviable) cells to produce red fluorescence. Cells that radiate orange/brown spectrum signifies apoptotic type of death, while necrotic LNCaP cells radiate red color. Viable cells were indicated by bright green color. Briefly, cell suspensions from each well of the treated MTP were transferred into identical small centrifuge tubes for centrifugation process. The overlying liquid layer was decanted and the cell pellets were re-suspended with PBS to make new cell suspensions for more analysis. Ethidium bromide (25μl) and Acridine orange (75μl) cocktail was made for coloring the cells. During the fluorescence microscopy, 3μL of the cocktail solution was added to 50μl each cell suspension (final dye-cell suspension). In order to examine the cells, 10-20μl of each dye cell suspension was transferred onto a microscope slide, covered with a cover slip and analyzed under a fluorescent microscope with a band pass filter (200X). Detection of apoptosis was based on morphological and fluorescent characteristics of stained cells.
The cells were treated with varying concentrations of (Gn0 - Gn70) for 24hrs. The linear regression analysis for each treatment showed that there was a negative correlation percent viability and treatment concentrations (P < 0.05). Data are the mean ± SD of two independent experiment performed in triplicate. Bar= SD (Figure 2). The growth and viability of LNCaP cells were assessed using the MTT assay. The cells were treated with varying concentrations of vitamin C (VitC0-70). The linear regression analysis for each treatment showed that there was a negative correlation between the values of absorbance and treatment concentrations (P < 0.05). Data are the mean ± SD of two independent experiments performed in triplicate. Bar= SD (Figure 3). Growth and viability of LNCaP cells were assessed using the MTT assay. The cells were treated with varying concentrations of genistein (Gn0- 70), Vitamin C (Vit C0-70 ) and genistein-vitamin C combination for 24hrs as described in the methods. Data are the mean ± SD of two independent experiments performed in triplicate. Bar=Standard Deviation (Figure 4).
The linear regression indicates the slope for single and combination treatment groups. The p-value of the linear regression performed = 0.11.The single treatment (Gn0-70uM) and combination treatment of genistein (Gn0-70uM + Vitamin 40uM) impacted the same level of anti-proliferative effect on LNCaP prostate cancer cells (Figure 5). The number of apoptosis, necrosis and living cells was counted from the microphotograph picture after florescence assay. Fifty cells per field were counted in three different fields and the average was calculated. The average was converted to percentage and the ratio of apoptosis to necrosis was calculated for the genistein treatment and the genistein-vitamin C combination treatments (Table 1). Apoptosis in LNCaP prostate cancer cells were assessed using the EtBr/AcrO florescence assay. The cells were exposed to varying concentrations of genistein (Gn0-70uM) for 24hrs at 37°C, 5% CO2. Data are the mean ± SD (Standard Deviation) of two independent experiments performed in triplicate. Bar=SD. The p-value of the chi-square for comparing mode of cell death was = 0.0003 (Figure 6). Percentage of Apoptosis in single and combination treatment. P-value of chi-square test comparing the single treatment of 70uM genistein with the combination treatment of Genistein 70uM + Vitamin C 40uM = 0.0164, at significance level of 0.05. This result further indicates that there is significant difference in the apoptotic induction in the single treatment and combination treatment (Figure 7).
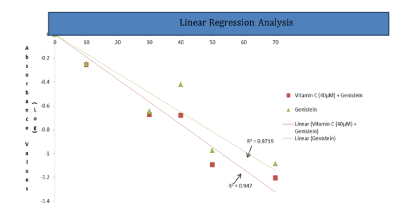
Figure 5 MTT results for treatment groups (vitamin C, genistein and genistein-vitamin C combination).
Treatment |
% Apoptosis |
% Necrosis |
% Apoptosis / % Necrosis |
Control |
|||
10uM genistein |
48 |
17 |
2.8 = 3 |
70uM genistein |
43 |
14 |
1.1= 1 |
10uM genistein +40uM vitamin C |
48 |
2 |
24.8= 25 |
30uM genistein+40uM of vitamin C |
48 |
4 |
12 |
70uM genistein +40uM of vitamin C |
62 |
6 |
10.3 = 10 |
Table 1 Ratio of apoptosis to necrosis in each treatment groups of LNCaP cells.
Note: % apoptosis to % necrosis is a method for comparing the effectiveness of single treatment of genistein and combination treatment (genistein + vitamin C) in impacting apoptosis on LNCaP prostate cancer cells
This picture depicts that there was more apoptotic cell death at70uM Genistein + 40uM Vitamin C than other concentrations. This is the late stage of apoptosis. The ratio of % apoptosis to % necrosis is 10 at this combination treatment. This figure indicates that there was more apoptotic cell death than necrotic death in the combination treatment (Figure 8). There was more necrotic death at 70uM genistein treatment as compared to 10uM genistein concentration. The ratio of % apoptosis to % necrosis is 1 for 70uM genistein treatment. This figure indicates that there was more necrotic death in the genistein treatment than in the combination treatment (Genistein + vitamin C) (Figure 9). The living cells in the control group were not treated and appeared green under the fluorescence assay. This figure shows that treatment of the cells is the cause of the apoptotic and necrotic death (Figure 10). This combination treatment has the highest % apoptosis to % necrosis ratio = 25. This combination treatment has fewer necrosis compared to the single treatment with 10uM genistein (Figure 11). Apoptosis is involved in genistein-induced Chemo-enhancement of LNCaP cell line. In order to confirm that apoptosis was induced by genistein and /or vitamin C at IC 50 concentrations of 40µM and 25µM respectively, LNCaP cells were analyzed in the presence of acridine orange/ethidium bromide staining (AO/EB staining). As a control, LNCaP cells were cultured in RPMI 1640 growth media and stained with AO/EB. Combination treatment of genistein and vitamin C at all concentrations induced apoptosis after 24 hours of incubation. Cells stained green represent viable cells, whereas yellow stained cells denote early apoptotic cell death stage. Cells with reddish stain depict that they have undergone necrosis. Genistein and/or vitamin C alter the intracellular levels of ROS and SOD in LNCaP cells: Intracellular levels of superoxide anion were analyzed using Nitro blue Tetrazolium (NBT)-ROS assay based on the mechanism of SOD inhibition. NBT reduction is used as an indicator of superoxide ion (free radical) production. When compared with the control treatment, all treatment regimens had decreased ROS levels, as seen in (Figures 12 & 13). Concisely, LNCaP cells was be seeded at 1.0× 104 cells per well in 96-well micro plates and allowed to attach for 24 hours at 37°C and 5% CO2. NBT (1mg/mL) in HBSS medium was added to the wells 24 hours after treatment, and incubated for 4 hours at 37°C in the dark. After incubation, cells were trypsinized and counted (Figure 12). Concisely, LNCaP cells were seeded at 1.0× 104 cells per well in 96-well micro plates and allowed to attach for 24 hours at 37°C and 5% CO2. NBT (1mg/mL) in HBSS medium was added to the wells 24 hours after treatment, and incubated for 4 hours at 37°C in the dark. After incubation, cells were trypsinized and counted (Figure 13).
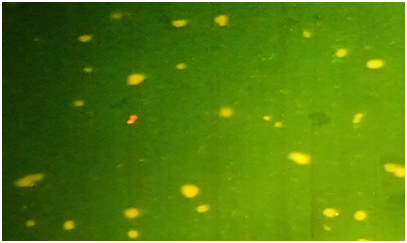
Figure 8 Microphotograph picture of florescence assay for combination treatment (70uM Genistein + Vitamin C 40uM vitamin C).
The MTT assay performed showed that, in terms of cytotoxicity, both treatments were not significantly different from one another. This shows us that the addition of vitamin C to a genistein-only treatment does not kill more LNCaP cells in vitro. The manner through which cancer cells die plays an important role in the clinical efficacy of these treatments, and this is why a fluorescence assay was performed. The acridine orange and ethidium bromide fluorescence assay performed show qualitative evidence of apoptotic, necrotic, and living cells present in the wells used for each treatment. Although apoptosis is the desired mode of cell death, mere observations of this are not enough to determine whether or not vitamin C has had a significant impact on the genistein treatment. Thus, a chi-square test was constructed based on the quantitative data obtained for each treatment measured. The chi-square test revealed that for the genistein-only treatment (70µM) versus the genistein (70µM) + vitamin C (40µM) treatment, a significant difference (P<0.05) did exist for apoptotic death between treatments. When vitamin C is added to a genistein treatment, significantly more cells will die by apoptosis. Thus, vitamin C does have an impact on genistein treatment. The nitro blue tetrazolium assay showed that there was a significant, negative correlation between the absorbance value percentages of superoxide and the concentration of the genistein-only treatment. There was also a significant, negative correlation between the absorbance value in percentages of superoxide and the concentration of the combination treatment. This shows that, compared to the control, the levels of SOD decrease in LNCaP cells as the concentration of the treatment increases.
This is significance because it shows that genistein as well as the combination of genistein and vitamin C had anti-oxidant effects on these cells. These results seem to contradict what has been found in previous studies. A possible reason for this discrepancy could be that the meaning of high dosage can be ambiguous. The interpretation of Figure 6 indicates that the combination treatment of genistein and vitamin C should be more efficacious than a genistein-only treatment (based on the proportion of apoptosis to necrosis). The chi-square test did show a significant difference in apoptotic death between the genistein-only treatment (70µM) and combination treatment (genistein 70µM; vitamin C 40µM). Necrotic death is smaller, and apoptotic death is significantly larger in the combination treatment; the number of living LNCaP cells present in the combination treatment is not smaller, however. This disparity in living LNCaP cells between the two treatments raises an important concern. As mentioned before, the addition of vitamin C to genistein will kill more LNCaP cells through apoptosis. The challenge of more living LNCaP cells in the combination treatment does raises the question of whether this negates its promotion as the superior treatment. This is a question that will have to be answered with future studies.
Combination treatment (Gn-Vit C) was more effective in apoptosis induction. These results indicate the potential clinical significance of vitamin C supplementation on genistein action as a chemo-preventive drug (Tsuda H et al. 2004).This study forms potential basis for in vivo studies of the impact of vitamin C on genistein-induced apoptosis in LNCaP cells.
My sincere gratitude goes to my committee members for all of their guidance and support, and special thanks to my adviser for his persistence, patience and encouragement during the writing of this manuscript. I am grateful to Department of Biological Sciences for providing the space to conduct this study. Finally, I appreciate the support of my co-authors and lab mates.
The authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.