Journal of
eISSN: 2373-633X


Prostate cancer is the most common non-cutaneous cancer and the second cause of cancer-related deaths in men in United State of America. An estimated 220,800 new cases and 27,540 cancer-related deaths are expected in the year 2015. Significant achievements in cancer treatment have been accomplished due to aggressive pursuit in therapeutic research. However, although the locally confined disease is treatable, the metastasized prostate cancer still poses therapeutic challenges; and consequently prognosis still remains poor. Inevitably, continuous research for efficacious therapy has been the focus of attention in recent times. In this study, the impact of vitamin C supplementation on the anticancer activities of genistein isoflavone in radio-sensitized LNCaP prostate cancer cells was investigated. The cells were first radio-sensitized with very low dose ionizing radiation (VLDR, 20mGy/hr), using a pyroelectrically generated X-ray source (non-radioactive) and analyzed using:
The overall data revealed that in all the three treatment regimens/modalities:
The overall result indicated that the triple combination treatment (Rad-Gn-VitC) was the most effect in apoptosis induction and ROS inhibition. The present study indicates the potential clinical significance of vitamin C supplementation in radiotherapy and radiation-genistein combination phytotherapy.
Keywords:vitamin C, prostate cancer, low-dose radio-sensitization, apoptosis, ROS, genistein
VLDR, very low dose ionizing radiation; NBT, nitroblue tetrazolium assay; HRS, hyper-radiosensitivity; DMSO, dimethylsulfoxide; MTP, microtiter plates; CSR, cell survival rate; DGIR, differential growth inhibition rate; ROS, reactive oxygen species
Despite the large investments in cancer researches and the wealth of knowledge and discoveries in cancer biology and genetics, prostate cancer is still at best, minimally controlled by modern medicine.1,2 The age adjusted mortality rate for cancer is about the same in the 21st century as it was 50 years ago. Prostate cancer is the most common non-cutaneous cancer and the second cause of cancer-related deaths in men of the United State of America, with nearly 1 out of every 7 men expected to be diagnosed with prostate cancer during their lifetime. An estimated 220,800 new cases and 27,540 cancer-related deaths are expected in the year 2015.3
Significant achievements in cancer treatment have been accomplished due to aggressive pursuit of research in therapeutic regimens. However, although the locally confined disease is treatable, the metastasized prostate cancer still poses therapeutic challenges; and consequently prognosis still remains poor. In addition to surgery, the standard chemotherapy and radiotherapy have been the most effective treatment regiments. But such therapies have their limitations in terms of safety, tolerability, and efficacy, partly due to incomplete elimination of the metastasized cancer and the serious side-effects resulting from treatment-induce toxicity.4 Development of alternative strategic therapeutic regimen has been the on-going focus of major research endeavors. Indeed, current approach in therapeutic research is focusing on attacking cancer cells at the molecular level in pursuit of signal transduction inhibitors, apoptosis-inducing molecules, and growth inhibitors since the 1990s.5 This means identifying specific cancer-associated biomarkers/proteins, growth promoting molecules, and signaling pathways within the cells that could be specific targets for therapeutic agents.
Several studies have documented the modulatory effects of genistein in prostate cancer carcinogenesis.6-9 Genistein isoflavone (4’, 5’, 7’-trihydroxy-isoflavone) is phytochemical polyphenol flavonoid micronutrients that have been found potent to possess strong anti-cancer activities.4,10 Genistein has been reported to induce cytotoxicity, inhibit cellular proliferation, and promote apoptotic cell death in various cancer cells in vivo and in vitro.11,12 The anti-cancer effects of genistein has been demonstrated in many human cancers such as breast, lung, prostate, lymphoma, leukemia, and head and neck cancers11,13 through many mechanisms that include: the inhibition of topoisomerase I & II,14 inhibition of telomerase activity,15 inhibition of tyrosine kinase signaling pathway, inhibition of cell survival proteins; Akt and NF-kβ16 and anti-apoptotic proteins -Bcl-2 and Bcl-XL,17-19 and also the inhibition of angiogenesis.10 Genistein has been shown to also display bipolar properties in which at low concentrations, it behaves as an antioxidant and at high concentration acts as a pro-oxidant.12
Vitamin C (Vit C or VtC) is another micronutrient of public health significance. Vitamin C, also known as L-ascorbic acid, is a naturally occurring potent antioxidant and cofactor for many enzymes. Due to the lack of enzyme L-gulonolactone oxidase, vitamin C must be obtained from food such as citrus fruits such as oranges, grapefruit, and lemons, and in green leafy vegetables, tomatoes, potatoes, strawberries, red or green peppers, and cantaloupe.20,21 The importance of this vitamin was first revealed by studies conducted by Linus Paulin in the 1970s.22 Vitamin C has been demonstrated to induce apoptosis in cancer cells by creating oxidative stress via upregulation of reactive oxygen species (ROS) release.23 Using high dosage of vitamin C as an anti-cancer therapy adjuvant has shown to reduce cancer cell growth and lessen chemo-therapy side effects such as nausea, fatigue, pain and depression.22
Of significance in therapeutic regimen is the application of low dose radiation. Clinical application of radiation indicates that many cancer cells are sensitive to low doses of radiation, and that very low doses can be used to augment or potentiate the chemotherapeutic effects of most drugs, while decreasing the development of resistance the by the cancer.24 At low radiation doses of less than 0.3Gy, hyper-radiosensitivity (HRS) has been demonstrated to be responsible for the increased sensitivity and decreasing chemo- and radio-resistance observed in these tumor cells.12,25-27
Previous studies in our laboratory revealed that exposure of LNCaP cells to very low dose radiation at 20mGy/hr followed by treatment with graded doses of genistein isoflavone induced significant apoptotic cell death with significantly less necrosis. The objective of this study was to access the potential impact of Vitamin C supplement on genistein-VLD radiation combination treatment of LNCaP prostate cancer cells. The objective of the current study was to investigate the therapeutic impact of vitamin C on the anticancer effects or activities of genistein isoflavone in radiation-sensitized LNCaP prostate cancer cells in vitro.
Drugs (Micronutrients)
Genistein (4’, 5’ 7- trihydroisoflavone) was purchased from Indoline Chemical Co., Summerville, NJ, USA. It was dissolved in DMSO (Dimethylsulfoxide) to make a 100μM stock solution. The final concentration of the DMSO solvent was 0.5%. Aliquots in concentrations of 0, 10, 20, 30, and 50µM (G0-G50) were made based on previous studies done in the laboratory4,12 and stored at 3°C until used.
Vitamin C supplement (Swanson, ND, USA) containing 100% ascorbic acid micronutrient was dissolved in distilled water to make a 100μM stock solution. Aliquots in concentration of 0, 10, 20, 30, 40μM (VtC0-50) from preliminary findings and was stored at 3°C until used.
Bioassays and staining reagents
MTT (3-[4, 5-dimethylthiazolyl-2]-2, 5-diphenyletrazolium bromide) assay, Nitroblue Tetrazolium (NBT) assay, and Acridine orange/Ethidium bromide (AO/EB) were purchased from Sigma-Aldrich, MO, USA. LNCaP prostate cancer cell lines were purchased from American Type Culture Collection-Manassas, VA. The cell lines were grown in RPMI 1640 complete medium (ATCC, VA) and maintained as monolayers in 75m2 tissue culture flasks (Sigma Scientific, St. Louis, MO, USA).
Cultivation of LNCaP cells
Briefly, LNCaP cells were plated in triplicates into 96-well microtiter plates (MTP) at a concentration of 2.5×104 cells per well. The plates were incubated in humidified CO2 incubator at 37oC and 5% CO2 for 48 hr to attain 80-90% confluence. The cells were then subjected to the following treatments.
Low dose X-ray irradiation
The cells in the MTPs were irradiated using a miniaturized portable pyroelectric X-ray generator (Cool X, Amptek Inc, Bedford, MA) based on the principle of pyroelectricity. Cells were irradiated with exposure to single doses of very low dose ionizing radiation (VLDR) at 20mGy/hour (i.e. 0.3mGy/min dose rate) low LET X-ray radiation.
Treatment of irradiated LNCaP cells
Immediately after irradiation, the cells were treated with:
From our preliminary study, the IC50 (i.e. the concentration of Vit C at which 50% of the cells were killed or inhibited) was observed to be 30µM. Thus in the present study, Vit C at concentration of 30µM (IC50) was used in the VtC-Gn combination treatment of LNCaP. The plates/cells were then incubated for 24 to 48 hours at 37°C and 5% CO2.
MTT assay
To determine the anti-proliferative or growth inhibitory effects of VLDR and/or genistein treatments on each prostate cancer cell line, MTT assay was used. MTT (3-[4, 5-dimethylthiazolyl-2]-2, 5-diphenyletrazolium bromide) is a tetrazolium dye used to determine percentage cell survival rate (% CSR) or differential growth inhibition rate (DGIR), which is equivalent to HRS in this study. The MTT dye detects metabolic activity through preferential conversion of viable cells into purple colored formazan. The enzyme mitochondria dehydrogenase is only present in the active mitochondria of living cells, and is responsible for cleaving the tetrazolium substrate into insoluble formazan. The color intensity generated is directly proportional to the number of viable (metabolically active cells) and quantitatively determined by optical absorbance from individual wells. Briefly, LNCaP and PC3 cells were plated onto 96 well plate each at a density of 2.5×104 cells per well. They were primed with single doses of 20mGy/hour ionizing radiation (VLDR) from a pyroelectric X-ray generator and then incubated with graded doses of genistein (Gn0-70) for 24 hours. Cells were treated with genistein, 4 hours after irradiation for most experiments. 100µL of MTT (2.5mg/ml in PBS-phosphate buffered saline) (Sigma Scientific Chemical Co., St Louis, MO, USA) solution was added to each well and incubated for 4 hours at 37°C and 5% CO2 to allow for the reduction of MTT into formazan by viable cells. The insoluble formazan crystals formed were solubilized or lysed to release the formazan product by adding 100μL of lysing solution (DMSO) to each pellet. Cells from each well were harvested (by vigorous aspiration with the micropipette), transferred to a labeled microcentrifuge tube and centrifuged for 10 minutes at 5000rpm. Any unincorporated MTT dye was removed by discarding the supernatant. The contents of each tube were transferred into different wells of a 96-well microtiter plate. The absorbance values were measured at 490nm with an automated microplate reader (BioTek, Vermont, USA). Relative numbers of live cells could therefore be determined based on the optical absorbance (optical density, OD) of the sample. The values of the blank wells were subtracted from each well of treated and control cells; and the mean percentage of post-treatment viable cells relative to the controls was calculated as shown:
Where AC is the absorbance of the control (mean value), AT is the absorbance of the treated cells (mean value), and AB is the absorbance of the blank (mean value).
The means of the absorbance were graphed against the mean concentrations of the drugs/reagents. All the microtiter plates were microphotographed under inverted microscope (200X) using digital camera (Nikon: Coolpix VR & ISO 2000, Japan), for histomorphological studies.
Nitroblue Tetrazolium NBT/ROS assay
Cells have many defense mechanisms against effects of reactive oxygen species (ROS). In this assay, superoxide ions (O2-) and/or hydrogen peroxides generated by X-rays and/ or graded genistein and or vitamin C treatments reduced or converted NBT to NBT diformazan. SOD reduces the superoxide ion concentration and thereby lowers the rate of NBT-diformazan formation. In this experiment, the treated LNCaP cells, as previously described above (with irradiation, Gn, Vit C) were subjected to NBT assay. The extent of treatment-induce reduction in the appearance of NBT diformazan is a measure of SOD activity present in the cancer cells. Since the absorbance at 490nm is proportional to the amount of superoxide anion (ROS) formed, the SOD enzyme as an inhibition activity can be quantified by measuring the decrease in the absorbance at 490nm, whereas an increase in absorbance reflects treatment-induced elevation of intracellular ROS produced by the cancer cells as demonstrated by Oseni et al.12
Briefly, 40μL NBT solution (1mg/mL in HBSS medium) was added to the adherent cells in each well in the appropriate MTP, and incubated for 4 hours at 37°C and 5%CO2. The cells were trypsinized, pipetted into individual micro-centrifuge tubes, and diluted with equal volumes of PBS. The cell suspensions were centrifuged to collect the pellets. The pellets were washed three times with DMSO to lyse the cell membranes. The supernatants were pooled for absorbance reading on microplate reader at 490nm, to determine ROS production levels. The results were expressed as percentage of ROS (or SOD inhibition), relative the control. The means of the absorbance were graphed against the mean concentrations of the drugs/reagents.
Fluorescence microscopy: Differential cell death detection
Acridine orange and Ethidium bromide dyes have different fluorescence emission spectra. Therefore a cocktail solution of the two markers can be used to differentiate between viable, apoptotic and necrotic cells. Acridine orange permeates both viable and non-viable cells, causing the nuclei to emit green fluorescence. Ethidium bromide permeates dead cells (with compromised cell membrane integrity) and selectively stains the nuclei (non-viable) cells to produce red fluorescence. Cells that emit orange/brown colors are indicative of apoptosis, while necrotic cells emit red fluorescence.
Briefly, cell suspensions from each well of the treated MTP were transferred into identical micro-centrifuge tubes for centrifugation. The supernatants were discarded and the cell pellets were resuspended with PBS to constitute final cell suspensions for further analysis. Then a cocktail of Ethidium bromide (25μl) and Acridine orange (75μl) was prepared for staining the cells. For the fluorescence microscopy, 3μL of the cocktail solution was added to 50μl each cell suspension (final dye-cell suspension). To examine the cells, 10-20μl of each dye-cell suspension was transferred onto a microscope slide, covered with a cover slip and analyzed under a fluorescent microscope with a band-pass filter (200X). Detection of apoptosis was based on morphological and fluorescent characteristics of stained cells. Viable cells were indicated by bright green color, apoptotic cells by yellow/orange/brown, and necrotic cells by red. Cell death was quantified by counting a total of 100 cells in various fields per slide. And the percentage of live cells, apoptosis, and necrosis were determined. Microphotographs were taken using a digital camera (Nikon: Coolpix VR & ISO 2000, Japan). The percentage of cells undergoing apoptosis were graphed against the mean concentrations of the drugs/treatment reagents. The correlation between treatment-induced ROS production and treatment-induced apoptosis was determined, using the data generated
Statistical analysis
Data were expressed as means+standard deviation (SD) from two different triplicate experiments to confirm similar result. The significance of the statistical difference in the mean differences between various experimental and control groups was determined using student’s t-test and one way ANOVA. P value of ≤0.01 was considered statistically significant.
To determine the impact of Vitamin C supplement on genistein-induced apoptosis in radiation sensitized LNCaP prostate cell lines, the cells were first irradiated with very low dose radiation (VLDR); and immediately subjected to genistein and/or vitamin C treatment. Treated cells were analyzed with various bioassays-MTT, NBT, and AO/ EB fluorescence microscopy, as described previously.
The MTT assay revealed a post-treatment dose-dependent survival rate in all the treatment regimens. The number of life cells declined significantly with increasing concentration of the drugs, and with significant inter-treatment differences (P<0.01) (Figure 1). The highest cell death occurred in the Gn-Rad-VitC combination treatment and the lowest in cells treated with Vit C alone.
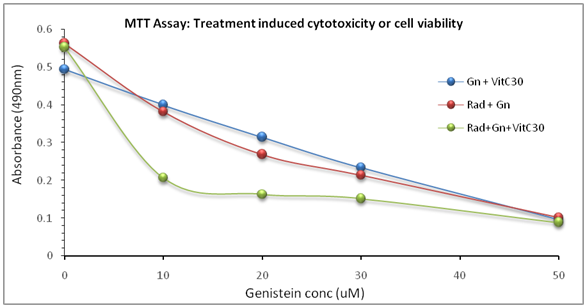
Figure 1 MTT assay result showing progressive decline in live cells with concurrent increasing concentration of the drugs. The results indicated statistical differences (P< 0.01) between the different treatment regimens at all concentration points. The results/data points were the means of two independent experiments performed in triplicates.
The NBT assay determined treatment-induced production amount of ROS in the treated cells. The results indicate dose-dependent ROS production in all the three treatment groups. There was significant inter-treatment differences (P<0.01) between the three treatment groups at every dosage level. The triple Gn-Rd-Vit C combination treatment has the highest inhibitory effects on ROS production/release (Figure 2).

Figure 2 NBT assay results showing treatment-induced inhibition of SOD/ROS production in LNCaP cells. Cells were treated as described and subjected to the NBT assay for ROS determination. The results indicated statistical differences (P<0.01) between the different treatment regimens at all concentration points. The results/data points were the means of two independent experiments performed in triplicates.
To assess and confirm treatment-induced apoptosis, the treated cells were processed and examined under fluorescence microscopy as previously described elsewhere in this manuscript.
The results/data obtained indicated dose-dependent treatment-induced apoptosis induction (Figures 3 & 4). At all dosage levels, the inter-treatment group differences were significant (P<0.01). It appeared vitamin C significantly augmented apoptosis induction in the combination treatment groups/cells (Figures 3 & 4). The data further indicated a significant negative correlation between treatment-induced apoptosis induction and ROS production (r=-0.83; r2=0.911; P<0.01) (Figures 5A & 5B). The results showed increasing percentage apoptosis concurrent with decreasing ROS production; implying treatment-induced ROS inhibition. The overall data showed the significant impact of vitamin C on apoptosis induction, as shown in the combination treatments relative to the control (Figures 1-5).

Figure 3A, 3B & 3C AO/EB Fluorescence assay results showing treatment-induced cell death in LNCaP cells. Cells were cultured and treated as described previously; then prepared and analyzed under fluorescence microscopy. Percentage life, necrotic and apoptotic cell deaths were determined. The results/data points were from two independent experiments performed in triplicates.
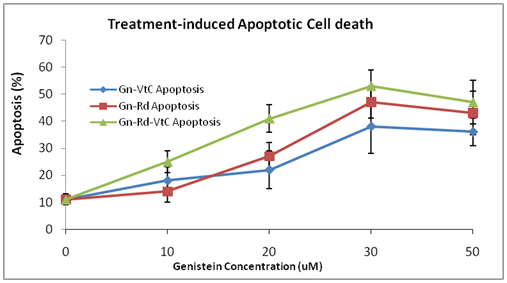
Figure 4 Treatment-induced apoptotic cell death in LNCaP cells. Cells were cultured and treated as described previously; then prepared and analyzed under fluorescence microscopy. The results/data points were from two independent experiments performed in triplicates. The bars are the STDs of the means. The % apoptosis at each concentration point are statistically different (P<0.01) between the three different treatment regimens.
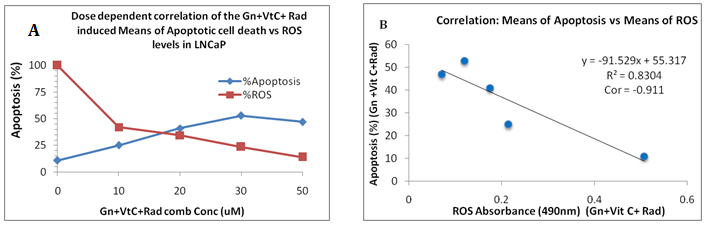
Figure 5A&5B The treated cells were subjected to treatment-induced apoptosis induction and ROS production as previously described. The results showed: A, as concentration increases, ROS production decreases and % apoptosis increases correspondingly and in Figure. 5B, there was a significant negative correlation (r =- 0.911; r2=0.83; P<0.01) between ROS production levels and apoptosis induction.
MTT assay is validated to assess the response of cancer cells including prostate cancer cells, to both radiotherapy and chemotherapeutic agents.28,29 In all the treatment groups, the MTT assay indicated a dose-dependent response of the cells to the various treatment regimens, in conformity with results of previous studies.10,12,14
The NBT assay results also revealed a dose-dependent decline in ROS production in all the three treatment regimens; with significant inter-treatment differences (P<0.01). Cancer cells produce high levels of ROS to survive oxidative stress conditions/environment.30-32 However, for cellular proliferation, these cells must maintain a balanced redox signaling system.12 An upregulation (overproduction) or down-regulation (underproduction) of ROS beyond the physiologic state may result in pathologic oxidative stress and consequent cell death/growth inhibition.31,33 Our present data, treatment-induced decrease in ROS production/down-regulation, is in conformity with these reported studies. In previous studies, we observed that lower genistein concentrations (<50µM) induced decreased ROS production and higher concentration (>50µM) induced increased intracellular ROS production.12 This is consistent with the current results, and the ROS modulation can be seen as one of the anti-cancer activities of genistein.
Genistein has been demonstrated to induce apoptosis cell death in many human cancer cells, including LNCaP and PC3 prostate cancer cells.10,12,14 The fluorescence microscopy study was done to assess the level of apoptosis cell death relative to concentration of drug in each treatment group. The results indicated that significant dose-dependent treatment-induced apoptosis occurred in LNCaP cancer cells; with significant differences (P<0.01) between the treatment groups. This result is consistent with previously reported data on genistein-induced apoptosis in human cancer cells.12,14
The combination-treatment studies demonstrate the potential therapeutic impacts of vitamin C and genistein on low dose x-ray radiation (20mGy/hr.) sensitized LNCaP prostate cancer cells. The highest percentage apoptosis induced in the cells was produced in the radiation-genistein-vitamin C combined treated cell group, followed by the radiation-genistein combined treated cells, and the least percentage of apoptosis was found in the single treatment cell group. These results are in conformity with previous studies and have also reinforced the claim that priming of prostate cancer cells with low dose ionizing radiation prior to treatment with genistein and/ or vitamin C can enhance their potential therapeutic or preventive effects. In all cases, radiation significantly augmented genistein-induced intracellular apoptotic cell death, in conformity with previously reported data.12,24,26,32 Furthermore, adding vitamin C as in the combination treatment, significantly augmented the genistein-induced apoptotic cell death, especially in the radiation-primed LNCaP cells. This observation highlights the potential significance of Vitamin C supplement in the treatment of cancer. The addition of vitamin C enhanced the highest percentage of apoptosis cell death in the present studies. The data/results from the present study indicate the potential clinical significance of vitamin C supplementation in radiation therapy and radiation-genistein phytotherapy; and genistein phytoprevention.34 Furthermore, the result/data justify further pursuit in this therapeutic strategy/regimen.
We acknowledge the Department of Biological Sciences of Florida Atlantic University for the provision of laboratory space. The technical support of the many research students in our lab is highly acknowledged.
Authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.