Journal of
eISSN: 2373-633X


Background: Controversy surrounds the use of prostate specific antigen (PSA) as a biomarker for prostate cancer detection, leaving an unmet need for a novel biomarker. In this regard, we investigated the association of two biomarkers, CCL11 and IL-6, with prostate cancer (PCa) and pointed out the diagnostic value of their combined detection and multiplication assessment.
Methods: In a cohort of 72 subjects (24 PCa; 24 benign prostatic hyperplasia (BPH); 24 healthy volunteers) serum concentrations of CCL11 and IL-6 were assessed by enzyme-linked immunosorbent assay (ELISA). Diagnostic performance of each biomarker and their multivariate assessments were compared using area under receiver operating characteristic curves (AUC) and the unpaired t-test.
Results: Mean serum concentrations of CCL11 and IL-6 were highly significantly (P<0.0001) elevated in PCa compared to controls. When CCL11 concentrations multiplied by those of IL-6, the yielded values were increased 2.7-fold and 6.1-fold in benign and malignant groups, respectively, as compared with normal group. Late stage tumors showed that ([CCL11]×[IL-6]) values (1544.0±176.0 pg/ml) were significantly (P<0.05) higher than those of early stage (665.8±68.5 pg/ml). The area under curve (AUC) was 0.933with superior sensitivity (96%) and higher specificity (75%).
Conclusion: We are here the first to report that values from combined detection and multiplication of (CCL11and IL-6) can be considered as novel promising bio-score biomarker for PCa detection and may be used also to distinguish between prostate enlargement and prostate cancer as well as its status. Our new score may be investigated to be surrogate to/or to be done with PSA.
Keywords: prostate cancer, cancer diagnosis, prostate biomarkers, CCL11, IL-6, PSA
BPH, benign prostatic hyperplasia; PSA, prostate specific antigen; PCa, prostate cancer; ELISA, enzyme-linked immunosorbent assay
Prostate cancer (PCa) is the second most common cancer in men, accounting for 10% of male cancers.1 The incidence of PCa is increasing in most Western populations.2,3 Today the only test that can fully confirm the diagnosis of prostate cancer is the transrectal ultrasound guided biopsy of 6-18 prostate cores in a patient, but this method is prone to sampling errors.4 Measurement of serum prostate specific antigen (PSA) is the most common tool used to detect prostate cancer.5 One of the problems of the PSA testing is that the biomarker itself has a weak correlation with degree of prostate malignancy; it is copiously produced by normal prostatic cells. So, lack of specificity and sensitivity limited its use as a screening tool in the general population.6,7 There is therefore still an urgent need for non-invasive biomarkers for the detection of PCa.
Chemokines are a super family of small secreted proteins initially characterized by their ability to induce leukocyte migration.8 During the transitions from normal to benign prostatic hyperplasia (BPH) and from BPH to PCa, a number of chemokines display variations in their expression.9 Several studies have shown that there are a potential link between CC-, and IL-type chemokines and cytokines as serum biomarkers and malignant proliferative diseases of the prostate.10-13 The current study was designed to investigate multivariate assessments of CCL11, a CC-type chemokine, and IL-6, an IL-type cytokine, and evaluate their diagnostic utility to distinguish between prostatic enlargements as BPH and PCa among Egyptian patients.
Patient population
The study was performed on 72 patients collected from the pool of patients referred to the Urology & Nephrology center seeking for treatment. Informed cancer was obtained from each patient who was included in the study .the studied patients were divided into 3 groups 24 patients each. Group1, had a confirmed diagnosis of prostatic cancer by transrectal prostate biopsy .Group 2 had the documented diagnosis of benign prostatic hyperplasia (BPH). Group 3 included 24 healthy individuals with no prostatic complaints and after complete investigations. The diagnosis of prostate cancer included tumor histology, tumor stage, lymphatic involvements, Gleason score and the evidence of radiological tumor metastases. The study protocol followed the ethical guidelines of the 1975 Helsinki Declaration.
Specimen processing
Six milliliters of blood from each subject were collected in vacutainer tubes (Becton Dickinson, Franklin Lakes, NJ) and were assigned a unique identifying number before delivery to the laboratory for processing. After collection, the blood was kept to retract for about 10 minutes. Each blood sample was then centrifuged at 1000×g for 10 minutes after which serum was decanted and aliquoted into cryovials and stored immediately at -20°C till analysis.
Laboratory analysis
Human CCL11levels (Cat# DTX00 R&D Systems Inc., Minneapolis, MN, USA) and human IL-6 levels (eBioscience, San Diego, CA, USA) in serum were monitored using ELISA. The assays were conducted according to the manufacturer’s instructions. The absorbance was read (Biotek, ELX800). Every sample was assayed in duplicate and the mean of two results was used. PSA serum levels were measured with an automated immunoassay analyzer (Axsyn, 7A83-97, Abbot).
Statistical analysis
All statistical calculations were done by a Statistical Package for the Social Sciences (SPSS); v.17.0 (SPSS Inc., Chicago, IL) and the GraphPad Prism package; v.5.0 (GraphPad Software, San Diego, CA). Patients characteristics were descriptive summarized and reported as mean±standard error of the mean (SEM). To test the significance of differences between the mean levels of a marker in different patient groups we used an unpaired t-test. Receiver operator characteristic (ROC) curves were generated and the area under the curve (AUC) tested for significance using an unpaired t-test against the hypothesis that the real area under the curve was 0.5 (i.e. no diagnostic value). The cut-off values for optimal clinical performance measures were determined from the ROC curves.
Clinical and pathologic characteristics
Prostate cancer staging was performed according to the TNM system of the American Joint Committee on Cancer (AJCC). Half of the patient had organ confined disease (T1, T2), while the remaining half had locally advanced tumor (T3, T4) with or without metastases. The patients were subdivided into two pathological groups according to the Gleason score: those who had Gleason score ≤7 in 14 patients while those who had >7 score were 10 patients. Other demographic and tumor characteristics are shown in Table 1.
Factor |
Value |
No. of Subjects |
24 |
Age (Years): Range (Mean±SD) |
59-81 (69.6±6.2) |
Tumor Histology: (Number) |
|
Adenocarcinoma |
21 |
Bone Metastatic Adenocarcinoma |
3 |
Pathological T Stage: (Number) |
|
≤T2 |
12 |
>T2 |
12 |
Grade: (Number) |
|
Well |
3 |
Moderate |
7 |
Poor |
11 |
Unknown |
3 |
Metastasis: (Number) |
|
+Ve |
12 |
- Ve |
12 |
Lymphatic Invasion: (Number) |
|
+Ve |
12 |
-Ve |
12 |
Gleason Score Categories: (Number) |
|
≤7 |
14 |
>7 |
10 |
Table 1 Clinical information of the prostate cancer patients
The ability of serum IL-6 and CCL11 in comparison with PSA to detect clinically significant PCa
Serum PSA, IL-6 and CCL11 were measured in 24 PCa, 24 BPH and 24 healthy individuals. The difference in serum concentrations of PSA, IL-6 and CCL11 in patients with and without PCa indicated potential diagnostic values. Figure 1 provides an array of ROC curves for PSA (Figure 1a), IL-6 (Figure 1b) and CCL11 (Figure 1c) to investigate their capability to distinguish between PCa and non PCa patients. The AUC calculated in this patient set was 0.865 for PSA, 0.870 for IL-6 and 0.880 for CCL11.
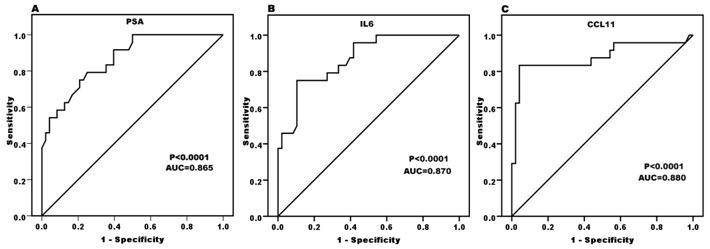
Figure 1 Discriminating prostate carcinoma patients from all non-cancer individuals: comparison of the ROC curves of PSA, IL-6 and CCL11. ROC, receiver operating characteristic; AUC, area under curve.
Figure 2 showed PSA for prostate cancer detection.
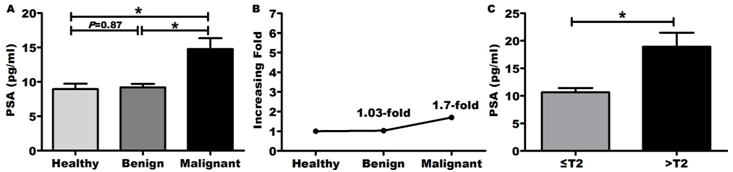
Figure 2 PSA for prostate cancer detection.
*P<0.05 is considered significant
IL-6 and CCL11 combination is predictive for PCa
Combining serum concentrations of IL-6 and CCL11 (IL-6+CCL11) increased the AUC to 0.900 (Figure 3a). This combination yielded values which were observed to be significantly higher in patients with PCa (197.4±14.3pg/ml) and BPH (124.5±4.7pg/ml) when compared with healthy control (97.8±1.1pg/ml, P<0.0001 for each comparison, Figure 3b). These yielded values were increased 1.3-fold and 2-fold in benign and malignant group, respectively, compared with normal control group (Figure 3c). Also, the values of this combination were increased significantly (P<0.0001) according to the stage of PCa (147.6±8.9pg/ml for early stages, 247.2±17.9pg/ml for late stages, Figure 3d).
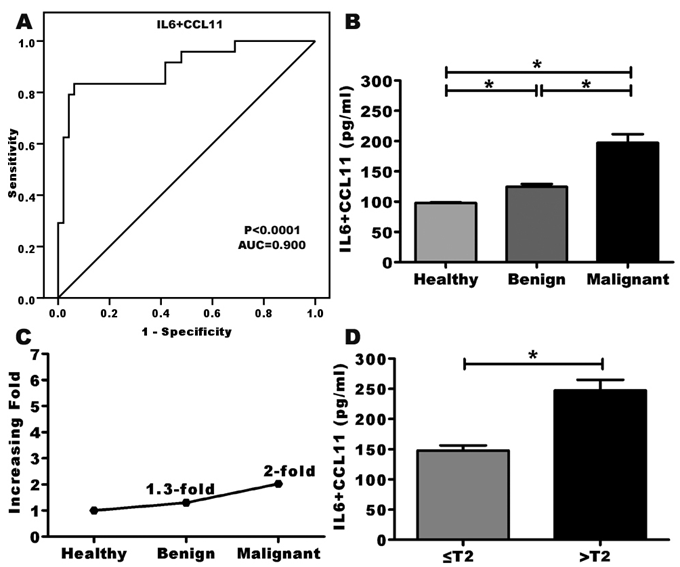
Figure 3 The combined use of IL-6 and CCL11 for prostate cancer detection.
*P<0.05 is considered significant
IL-6 and CCL11multiplication is most predictive of PCa
A multivariate assessment of these variables determined that the multiplication of IL-6 with CCL11 (IL-6×CCL11) was most predictive of prostate disease outcome. This multivariate test system to detect PCa produced an ROC curve with an AUC 0.933 (Figure 4a). This multiplication increase the power to discriminate PCa (1105±130.0 pg/ml) from BPH (484.5±42.0 pg/ml) and healthy (182.5±11.0 pg/ml) individuals (Figure 4b). Interestingly, serum (IL-6×CCL11) level was 6.1-fold and 2.7-fold higher in PCa and BPH, respectively than healthy control (Figure 4c). When comparing only the different stages of PCa, the serum (IL-6×CCL11) levels increase significantly (P<0.0001) as the stage increases (early stages (665.8±68.5 pg/ml), advanced stages ((1544.0±176.0 pg/ml), Figure 4c).

Figure 4 Multiplying IL-6 with CCL11 for prostate cancer detection.
*P<0.05 is considered significant
Sensitivity and specificity for investigated parameters in detection of PCa
The benign and healthy normal groups were combined in a non-malignant group, and the best cutoff values for investigated parameters were calculated by the ROC curve as 9ng/ml for PSA, 3.6pg/ml for IL-6, 116pg/ml for CCL11, 120.3pg/ml for IL-6+CCL11 combination and 418.5pg/ml for IL-6×CCL11 multiplication. Sensitivity and specificity for each serum marker as well as their combination and multiplication were tested for detection of PCa. Combining IL-6 and CCL11 increased sensitivity and specificity. The best sensitivity was achieved when the serum IL-6 multiplied with CCL11 (Table 2).
Marker |
Cutoff |
Sn (%) |
Sp (%) |
PPV |
NPV |
Ac (%) |
PSA |
9ng/ml |
79 |
73 |
61 |
88 |
76 |
IL-6 |
3.6pg/ml |
75 |
73 |
60 |
86 |
75 |
CCL11 |
116pg/ml |
82 |
73 |
61 |
90 |
76 |
IL-6+CCL11 |
120.3pg/ml |
83 |
75 |
61 |
90 |
76 |
IL-6×CCL11 |
418.5pg/ml |
96 |
75 |
64 |
97 |
81 |
Table 2 Ability of serum biomarkers in predicting the presence of prostate cancer
Sn, Sensitivity; Sp, Specificity; PPV, positive predictive value; NPV, negative predictive value; Ac, accuracy
Prostate cancer is the most frequent malignancy in men in the Western world.14 This cancer is mainly detected by the determination of serum PSA. However, several studies showed the limited ability of PSA in differentiating between benign and malignant prostate diseases and between aggressive and insignificant tumors since a continuous risk of prostate cancer occurs at all PSA values.15,16 Thus there is a need for new PCa diagnostic biomarkers.
Chemokines are part of the network of inflammatory mediators associated to neoplasia irrespective of pathogenesis.17 Recently among several chemokines studied in PCa, CCL11, a potent chemotactic factor for eosinophils, actually demonstrated elevated levels in PCa.1,10,18 On the other hand IL-6 is a proinflammatory cytokine that plays an important role in intraprostatic inflammation and thus carcinogenesis.19 In this regard, we aimed to estimate the diagnostic performance of the combined use of CCL11, IL-6 and those of each separately compared with PSA in PCa detection. Using ELISA, we examined CCL11 and IL-6 serum levels in patients with biopsy and clinically confirmed BPH or various stages of PCa. Increasing concentrations of both CCL11 and IL-6 positively correlated to tumor burden and identified patients with all stages of PCa. This may be explained by the fact that suggested by previous pathological studies that inflammation is implicated in prostate carcinogenesis.20,21 Therefore, proinflammatory cytokines (include CCL11 and IL-6) influence prostate cancer risk and the local production of these chemokines by the tumor can also result in increased chemokine concentration in the blood, as reported for patients with breast and gastric carcinoma.18,22,23
In a first attempt to unravel the diagnostic utility of the combined detection of CCL11 and IL-6 in PCa, CCL11+IL-6 revealed higher diagnostic value (AUC of 0.900) at a 120.3pg/ml cutoff with higher sensitivity (83%) and specificity (75%) for detecting the presence or absence of PCa. Interestingly, the highest diagnostic ability (AUC=0.933) was achieved when serum CCL11 concentrations were multiplied by those of IL-6 with sensitivity of 96% and specificity of 75%. Moreover, late stage tumors were associated with higher mean (CCL11×IL-6) values than early stage tumors (P<0.05). This compared favorably with results revealed from using PSA alone (sensitivity 79%, specificity 73% and AUC=0.865). In other analysis, the estimated sensitivity of a PSA cutoff of 4.0ng/mL was 21% for detecting any prostate cancer and 51% for detecting high-grade cancers.24
Future studies should focus on the prospective analysis of these markers against other established serum markers of prostate cancer in larger multi-centric studies. However, this approach is beyond the scope and limited financial resources of the present work. Taken together, our study highlights–for the first time- the potential utility of the combined use of CCL11 and IL-6 as novel diagnostic bio-score biomarker for PCa using a multiplication of serum value levels of both CCL11 and IL-6. We recently showed another equation in different type of cancer for the first time the impact of serum epithelial membrane antigen (EMA) and cytokeratin-1 (CK1) ratio in breast cancer detection, and showed that EMA/CK1 values may serve as diagnostic marker in early stage breast cancer.25
None.
Authors declare that there is no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.