Journal of
eISSN: 2469 - 2786


Conceptual Paper Volume 4 Issue 2
1School of Life Sciences, Shaanxi Normal University, China
2Lab of biochemical technique and neurosciences, Shaanxi Normal University, China
Correspondence: Javed Iqbal, School of Life Sciences, Shaanxi Normal University, Xian, P.R China
Received: November 03, 2016 | Published: March 8, 2017
Citation: Iqbal J, Ahmad R. Western blot: proteins separating technique, protocol, theory and trouble shooting. J Bacteriol Mycol Open Access. 2017;4(2):62-67. DOI: 10.15406/jbmoa.2017.04.00088
Western blotting is used to visualize proteins that have been separated by gel electrophoresis. It is an important technique used in cell and molecular biology. Specific proteins can be identified from a complex mixture of proteins extracted from cells. The western blot includes three major steps:
This technical article will attempt to explain the technique in detail to help researcher in getting good results and theory behind western blot, and offer some ways to troubleshoot.
Keywords: molecular biology, proteins, antibody, gel electrophoresis, western blot, phosphate buffered saline, spectrophotometer, solidification, electro phorator, protein extraction, antibody, protocol, protease degradation, horse radish peroxidase, positive electrode, blotting membrane, concentration
PBS, phosphate buffered saline; PVDF, poly vinylidene fluoride; BSA, bovine serum albumin; HRP, horse radish peroxidase
Western blot is used in molecular and biochemical research to separate and identify the proteins of interest. This technique involve separation of mixture of proteins based on molecular weight, and thus by type, through gel electrophoresis. The results on gel are then transferred to a membrane producing a band for each protein. The membrane is then incubated with labels primary and secondary antibodies specific to the protein of interest. The unbound antibody from the membrane is washed off leaving only the bound antibody to the protein of interest. The bound antibodies are then detected by developing the film. As the antibodies specifically only bind to the target protein, only one band should be visible. The thickness of the band corresponds to the amount of protein present in the sample; thus doing a standard can indicate the amount of protein present. This paper will first describe the protocol in detail for western blot, accompanied by theory to rationalize the protocol and followed by the theoretical explanation of the procedure, and in the later section, troubleshooting tips for common problems.
Protein extraction
Protein can be extracted from different kind of samples, such as tissue or cells. Below is the protocol to extract proteins from adherent cells.
Adherent cells:
Sample preparation
Reagents |
10% gel |
5% gel |
dH2O |
2.925ml |
2.720ml |
30% Acr/Bris |
2.550ml |
0.68 |
Tris (PH8.8 1.5m) |
1.875ml |
0.520ml (PH 6.8) |
10% SDS |
75ul |
40ul |
10% AP |
75ul |
40ul |
TEMED |
6ul |
4ul |
Table 1 Preparation of gel
Gel electrophoresis
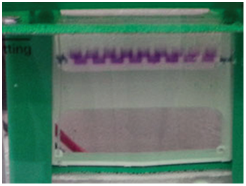

Figure 5 (a) Samples running through the stacking gel (lower voltage) (b): Samples running through the separating gel (higher voltage).
Electro transfer from gel to PVDF membrane
(Tip: Ensure there are no air bubbles between the gel and PVDF membrane, and squeeze out extra liquid).
Blocking and antibody incubation
*TBST: Tris-Buffered Saline Tween-20
Recipe
Thus, putting it in BSA solution allows the antibody to be reused it if the blot does not give good result. The antibody can be diluted in a wash buffer, such as PBS or TBST. Washing is also very important step as it minimized background and removes unbound antibody.
Sample preparation
Western blot depends on cell lysates which is the most common form of sample used in this technique. Protein extraction should be done in a cold temperature with protease inhibitors to collect all the proteins in the cell cytosol and prevent denaturing of the proteins. Since tissue sample have a higher structural organization, therefore mechanical invention such as sonication is needed to extract the proteins. After extracting the protein, the next important step is to have a good idea of the extracted proteins concentration. This allows the researcher to ensure that the samples are being compared on an equivalent basis.
Generally spectrophotometer is used for measuring the protein concentration. This concentration value is used to measure the mass of the protein that is being loaded into each well by the relationship between concentration, mass, and volume.
After determining the appropriate volume of the sample, it is diluted with loading buffer having tracking dye so that the samples sink easily into the wells of the gel. A tracking dye (bromophenol blue) allows the researcher to see how far the separation has progressed. In order to denature the higher order structural organization of protiens, while retaining sulfide bridges, the sample is heated after being diluted into a loading buffer. Denaturing ensures that the negative charge of amino acids is not neutralized, enabling the protein to move in an electric field (applied during electro transfer). It is important to use positive and negative controls for the sample. For a positive control a known source of target protein, such as purified protein or a control lysate is used to confirm the identity of the protein, and the activity of the antibody. A negative control is a null cell line is used to confirm that the staining is not nonspecific in nature.
Gel electrophoresis
Two different types of agarose gel named as: stacking and separating gel are used in western blot. The stacking gel is slightly acidic having pH 6.8 and has a lower acryl amide concentration making a porous gel, which separates protein poorly but allows them to form thin, sharply defined bands. The separating, or resolving gel, is basic with pH 8.8, and has higher poly acryl amide content, making the gel’s pores narrower. The smaller proteins are separated in this gel rapidly than larger proteins. The proteins loaded on the gel have a negative charge and will travel toward the positive electrode when a voltage is applied. Gels are usually made by pouring them between two glass and plastic plates, using the solution described in the protocol section. The wells are loaded with the samples and markers. Then, the gel is connected to the power supply and allowed to run. It is very important to regulate the voltage, as a high voltage can overheat and distort the bands on the gel, so care should be taken.
Blotting
After separating the protein mixture on the gel, the next important step is to transfer it to a membrane. This transfer is done using an electric field oriented perpendicular to the surface of the gel, causing proteins to move from gel to PVDF membrane. The membrane is placed between the gel surface and the positive electrode in a sandwich manner. The sandwich includes a sponge membrane at each end, and filter papers to protect the gel and blotting membrane Figure 12. During this process, two things are very important:
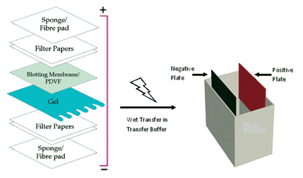
Figure 12 Assembly of a sandwich in western blot (transfer of protein bands from gel to PVDF membrane).
The membrane must be placed as such that helps the negatively charged proteins can migrate from the gel to the membrane. This type of transfer is called electro phoretic transfer, and can be carried out in semi-dry or wet conditions. Wet conditions are usually more favorable and reliable as it is less likely to dry out the gel, and is preferred for larger proteins.
The membrane used during transferring of proteins from gel to membrane is an essential part of this process and are of two types: nitrocellulose and PVDF. Nitrocellulose is used for its high affinity for protein but does not allow the membrane to be used for re-probing. In this regard, PVDF membranes provide better reliable support and allow the blot to be re-probed and stored. However, in case of PVDF membranes the background is higher and therefore, washing is very important step for this.
Washing, blocking and antibody incubation
Washing and blocking are very important steps of western blotting, as it prevents antibodies from binding to the membrane nonspecifically. Blocking is done with 5% BSA or nonfat dried milk diluted with TBST to reduce the effect of background. Nonfat dried milk is often preferred as it is inexpensive and widely available. However, care must be taken to choose the appropriate blocking solution because milk proteins are not compatible with all detection labels. For example, BSA blocking solutions are preferred with biotin and AP antibody labels, since milk contains casein, which is itself a phospho protein and biotin, thus interfering with the assay results. It is a good strategy to incubate the primary antibody with BSA since it is usually needed in higher amounts than the secondary antibody,
Using the label antibody usually with an enzyme such as horseradish peroxidase (HRP), the membrane is then detected by the signal. This signal is captured on a film which is usually developed in a dark room.
Quantification
It is very important to note that the data produced with a western blot is typically considered to be semi quantitative. It provides a relative comparison of protein levels, but not an absolute measure of quantity. There are two reasons; first, there are variations in loading and transfer rates between the samples in separate lanes which are different on separate blots. These differences will need to be standardized before a more precise comparison can be made. Second, the signal generated by detection is not linear across the concentration range of samples. Thus, since the signal produced is not linear, it should not be used to model the concentration.
Troubleshooting and suggestions
Western blot seems to be very simple technique but many problems can arise, leading to unexpected results. The problem can be grouped into five categories:
Unusual or unexpected bands can be due to protease degradation. In this case it is desirable to use a fresh sample which had been kept on ice or alter the antibody. Similarly, blurry bands are often caused by high voltage or air bubbles present during transfer. In this case, it should be ensured that the gel is run at a lower voltage, and that the transfer sandwich is prepared properly. Finally, white (negative) bands on the film are due to too much protein or antibody.
No bands can also arise due to many reasons related to antibody, antigen, or buffer used. The following factors are responsible for that;
Western blot is a protein detection technique as it allows the user to detect and quantify the protein expression as well. This technical paper covered the basic protocol, the theory behind that protocol, and some troubleshooting techniques and suggestions. It will help researcher to find protein expression accurately.
None.
The author declares no conflict of interest.

©2017 Iqbal, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.