Journal of
eISSN: 2469 - 2786


Research Article Volume 12 Issue 1
Department of Plant Science and Biotechnology, University of Jos, Nigeria
Correspondence: Michael Sila, Department of Plant Science and Biotechnology, University of Jos, Jos, Nigeria
Received: December 26, 2023 | Published: January 9, 2024
Citation: Sila MD, Nyam DD, Shutt VM, et al. The influence of moisture content migration and sugar concentrations in fungal biodeterioration of stored sweet potato (Ipomoea batata (L) lam) root tubers of Jos and environs. J Bacteriol Mycol Open Access. 2024;12(1):1-8. DOI: 10.15406/jbmoa.2024.12.00362
Harvested sweet potato (Ipomoea batatas (L) Lam) root tuber loss on the Jos Plateau is attributed to fungal biodeteriogens. Five improved cultivars: CIP4400168, Ex-Igbariam, Tanzania, TIS 8164 and TIS 87/0087 were collected, propagated, harvested and stored in a barn. The fungal colonisers, sugar concentration, moisture content migration within the cultivars circumference were investigated. Standard methods were used to identify the biodeteriogens and those that were amylolytic in activity. Physico-chemical analyses of the peels and five layers (depths) from it within each cultivars circumference were determined. Depths of penetration of the circumferences by the colonisers were monitored and each was plated out on standard media, divided into 3 batches and incubated at 250C, 370C and 450C. A total of 30 species of fungi: 3 Ascomycetes, 22 Hyphomycetes and 2 Phycomycetes, composed of 2 species of thermophiles (Mucor pusillus lindt and Scytalidium thermophilum Austwick), 4 species of thermotolerants and 24 mesophiles were identified. The genus Aspergillus had the highest representation; 3 yeasts: Candida albicans Berkhout, Rhodotorula species and Saccharomyces cerevisiae Hansen were also isolated. A. niger, A. oryzae, A. terreus, E. nidulans and Mucor pusillus were amylolytic in activity. Prior to storage Tanzania had peel MC of 49.60% and in-depth (5th layer) MC of 52.55%, but had 31.52% peel MC and 45.36% in-depth MC after 8 weeks of storage indicating that MC migration was directed towards its peels. Similar MC was recorded for the other cultivars after 2, 4, 6 and 8 weeks of storage. Ex-Igbariam peel prior to storage had the highest glucose content of 11.98% and an in-depth content of 0.21% as compared to CIP 4400168 which had 7.51% peel glucose content and in-depth content of 6.41% but after 8 weeks of storage, the former had 14.71% peel glucose content while the letter had increased to 9.67% glucose content. Similar tread in terms of greater peel glucose content were recorded for the other cultivars after 2, 4 and 6 weeks of storage. In terms of penetration of the decayed cultivars, 33 fungal colonisers were isolated on the peel with the highest concentration of glucose; 19, 14 and 2 fungal species were isolated from the second, third and the central layers respectively. This study has shown that the deteriorative and degradative activities of fungi during the cultivars storage were influenced by moisture content migration that mobilised glucose from the inner layers and concentrated it on the peels, encouraging proliferation and growth of the decaying biodeteriogens thereby reducing their shelf-life. To increase the shelf-life of the harvested cultivars plant extracts are being experimented with to control their enzymatic browning and to formulate sweet potato starch that could be utilised in food, pharmaceutical and textile industries and flour in bread, biscuit and cake baking.
Keywords: biodeteriogenics, moisture content (MC), enzymatic browning, depths (slices), peels (cultivars surfaces)
Most serious causes of loss in the root tubers of harvested sweet potato (Ipomoea batatas (L) Lam) on the Jos Plateau are attributed to fungal biodeteriogens.1 The organisms are distributed in the air, soil and in storage to constitute storage fungi.2–4 A wide range of fungal species which cause different types of decay in sweet potato root tubers originate from different sources.5,6
Food security and sustainability is one of the ways of addressing food scarcity and shortage due to the activities of fungi.7–9 On the Jos Plateau the sweet potato root tuber is an effective and economic source of energy and other nutrients, however the shelf-life of the root tuber is seriously hampered by decaying fungal activities which are encouraged by the root tubers moisture content and sugar concentration.8,10
The root tubers become moister and sweeter in taste after harvest, high in moisture content ranging 62.58–64.34, dry matter is low; carbohydrate consist mainly of starch; less pectin, hemicellulose and cellulose.11–13 Sweetness in the root tubers is due to the presence of endogenous sugars: sucrose, glucose, fructose at harvest and maltose formed through starch hydrolysis by amylase during storage or through heat treatment; raffinose, stachyose and verbascose were the other oligosaccharides assayed in the root tubers.9,14,15
In Jos, the root tubers compete favourably with Cassava, Yam and Taro in caloric content.1 The produce has extensive cultivation at the home steads. They are harvested and transported to the urban markets of Jos, get glutted due to poor patronage during retailing. Under market conditions, the commodity has a shelf life of 1-2 weeks thereafter become hardened, develop sprouts during storage and finally become decayed due to biodeteriogens. Development of secondary products such as sweet potato starch and flour with longer shelf-life than the wholesome root tubers could be a way of ameliorating the deterioration of the commodity and to expand utilization among the peasant farmers and consumers (industrialists).
In many food- deficit countries like Nigeria the need to fully utilize all existing foodstuffs with a view to alleviating poverty and hunger is now receiving considerable attention.16 One way of minimising post-harvest losses and to increase the utilization of sweet potato on the Jos Plateau is through processing the root tubers into secondary products with longer shelf-life.
The critical operation will reside in the root tubers control of enzymatic browning which reduces the value of its carbohydrate.17,18 Factors such as moisture content and sugar concentration of the root tubers that encourage their decay were analysed to ascertain the degree of biodeterioration. Therefore the objective of this study was to analyse the effects of moisture content migration and sugar concentrations at different depths of the stored root tuber circumference and to ascertain the biodeteriogenics at each depth.
The plot of land at Rayfield
The experimental plot of land measuring 45m×25m at Rayfield; an environ of Jos town; 9.20 North Latitude, 8.90 East Longitude and 1208 meters elevation above sea level was used for the cultivation of the root tubers. Jos is on the North and East hemispheres with cool temperature, fluctuating between 34.50 – 130C (GPS Coordinates of Jos and environs, July 2023). The cool temperature encourages the production of both temperate and tropical crops.
The plot is composed of soil that is adequately drained, pH of 5, organic content of 4%, and moisture content of 50%, receives adequate rays of sunshine during wet and dry seasons of the year and highly suitable for the cultivation of the root tubers. Therefore five improved cultivars: CIP4400168, Ex-Igbariam, Tanzania, TIS 8164 and TIS 87/0087 popular sweet potato root tubers on the Jos Plateau were collected from sweet potato farmers within nine local government areas (LGA) and were propagated on the farm.
Biodeterioration studies on the stored root tubers
Construction of the root tuber barn
The storage method of Okwuowulu and Osiegbu was modified to construct an In-door storage barn. An exclusive space, 4m x 1m was marked within an empty room with 2 windows gauzed with plastic net. The floor was washed, filled with fine sand and ash to a height of 4.00cm. Stones of equal dimensions were placed on the corners and middle of the area; wooden planks of equal length were laid on top of the stones to provide a flat surface area; ply-woods of 1.0m height were used to fence the demarcated area.
Fresh cultivars of 100Kg each was weighed out and were put in plastic baskets with wide opening on top and perforations on the sides. These were placed on the flat surface of the barn and were examined for fungal colonies fort-nightly for 8 weeks. The entire space between the rows of the baskets and the walls of the ply-wood was filled with Digitaria straw to prevent rapid moisture content loss of the root tubers. The entire room provided the shade which kept the barn cool as well as a wind break and was adopted for an in–door storage barn, allowing air to circulate freely within the root tubers without interference of humans and rodents.
Microbial isolation and purification of the stored decaying root tubers
The mycelia fungi and yeast colonies that developed were subjected to culturing and series of sub culturing using Malt Extract Agar (MEA) and Sabouraud Glocose Agar (SGA) respectively until pure cultures were obtained.
Identification of the isolates
The fungal isolates were subjected to microscopic examination in order to determine their identities. The cultural characteristics and morphological parameters of such fungal isolates were taken into consideration. References were made to fungal stock cultures and to.19–23 Also the keys of Klich24 were used to further identify the Aspergilli and Rhizopus species. The yeast isolates were examined microscopically and various biochemical tests such as India ink (wet preparation) test, Urease test, Fermentation test and Sugar assimilation test were carried out to finally identify the unicellular organisms.
Amylase production by some of the fungal isolates
Some of the fungal isolates from the decaying sweet potato root tubers that exhibited high percentages of occurrence such as Aspergillus niger, A. oryzae, A. terreus, Emiricella nidulans and Mucor pusillus and those conventionally employed for amylolytic studies were investigated on their abilities to produce amylase. The enzymatic study was centred on amylase production because the root tuber starch content is high ranging between 79.43 – 89.76%12 especially the peels.9 The species were cultured on starch agar for 7 days, the mycelia were scraped and surfaces of the culture plates were flooded with Lugol iodine solution.
The hydrolyzed part of the starch agar as a result of amylolytic activity was supposed to be colourless, the non-hydrolyzed portions were supposed to be blue-black. Positive result confirmed the ability of such test fungus (isolate) to produce amylase and therefore its ability to utilize the starch component of the root tuber, hence causing its deterioration.
Physico-chemical determinations of the stored root tubers
Determination of moisture content (MC) on wet weight basis
Percentage MC on wet weight basis of the peels and 5 different layers (depths) of the stored root tubers slices were determined fort-nightly. Each root tuber was washed, peeled; the underlining flesh was held in a longitudinal position on top of a chop board then sectioned into 5 layers (depths), each 5cm thick towards the centre of its circumference. The peels and the 5 layers (depths) were chopped into chips (slices) which were analysed for moisture content as follows:
Each experiment was replicated, the average values were recorded and analysed.
Quantitative determination of glucose on wet weight basis
Also the glucose concentration of the peels and the 5 different layers (depths) of the stored root tubers were determined fort-nightly. The wet chips of the peels and the 5 layers (depths) of each root tuber circumference were crushed and the sugar (glucose) content of the supernatant was determined on wet weight basis using a sensitive Glucose Oxidose Method (GOM). The values of the absorbance obtained were used for calculating the concentration of glucose in each sample by applying the Beer-Lambert’s formula:
Where: = Absorbance of sample’s glucose
= Absorbance of standard’s glucose
Each experiment was replicated, the average values were recorded and analysed.
The effects of storage on moisture content migration and sugar concentration at different layers (depths) of the stored root tubers circumference
Several studies had indicated that glucose content of the sweet potato root tuber was dispersed within its circumference. The glucose content of the stored root tubers was analysed fortnightly to determine the effects of this parameter on their colonization by decay fungi. Each cultivar was analysed for percentage moisture content and glucose concentration within its circumference. The percentage moisture content of the surface (peel) and the 5 layers (depths) of each stored root tuber to the centre of the circumference were monitored to determine its moisture content and directional loss (migration). It was also carried out to determine its effect on the root tuber glucose distribution either towards the outer or centre of its circumference and its relationship in terms of the number and species of fungi that colonized them especially the decaying ones.
Total reducing sugar (Glucose) of the stored root tuber’s surfaces (peels) and the 5 layers (depths) of the circumference was also routinely analysed to monitor the concentration of the glucose. It was carried out to determine whether the moisture content loss had effect on the glucose concentration of the surfaces (peels) and the 5 layers (depths) of the stored root tubers circumference which might have encouraged their colonization by decay fungi.
Tests on the depth of penetration of the root tubers circumference by the colonisers
In this experiment, the decaying root tubers were sliced into various layers from the surface. The peel constituted the first layer which was numbered (1). The following layer from the surface was (2). The third layer from the surface was numbered 3, then the fourth layer 4, 5th layer 5, 6th layer 6 and the final layer (to the centre) of the decaying root tuber 7.
Each of the resultant layers (depths) was cut into pieces of 1 × 1cm aseptically and was plated out on Malt Extract Agar (MEA), Czapek Dox Agar (CZA) and Sabouraud Glucose Agar (SGA). The resultant culture plates were again divided into 3 batches and incubated at 250C, 370C and 450C for the isolation of mesophilic, thermo tolerant and thermophilic fungi.8,9 The various fungal colonies were subcultured several times to obtain pure cultures which were duly identified as already described. Control plates that did not contain the root tuber slices were also prepared and incubated at the various temperatures for comparison.
The experiments were carried out in order to determine the depth of penetration of the biodeteriogens into the decaying root tubers and the levels of the layering that had the highest species. It was performed in order to show whether there was any relationship between the colonizing fungi and the sugar constituent of the various layers of the root tubers circumference.
A total of 30 species of fungi were isolated from the five stored decaying sweet potato tubers. Ex-Igbariam, Tanzania and TIS 8164 were more deteriorated by the species than CIP 4400168 and TIS 87/0087. Twenty one (21) of these species were isolated from Ex-Igbariam and Tanzania while twenty (20) and nineteen (19) were isolated from TIS 8164 and TIS 87/0087 respectively and the least number of nineteen (19) fungal species from CIP4400168 (Table 1).
|
Decaying sweet potato root tubers |
||||||
|
Fungi isolates |
CIP |
EX |
TAN |
TIS 81 |
TIS 87 |
Total |
|
Ascomycetes |
||||||
|
Emericella nidulans (Eldam) Vuill |
+ |
+ |
+ |
+ |
+ |
5 |
|
Eurotium amstelodami Mangi |
- |
- |
+ |
+ |
- |
2 |
|
E. harbarioram (Wiggers) Link |
+ |
- |
- |
+ |
- |
2 |
|
Hyphomycetes |
||||||
|
Aspergillus candidus Link |
+ |
+ |
+ |
- |
- |
3 |
|
A. clavatus Desm |
+ |
+ |
+ |
- |
+ |
4 |
|
A carneus Blochwitz |
- |
- |
+ |
+ |
2 |
|
|
A. flavus link ex. Gray |
+ |
+ |
+ |
- |
+ |
4 |
|
A. fumigatus Fres |
+ |
+ |
_ |
+ |
+ |
4 |
|
A. niger van Tieghem |
+ |
+ |
+ |
+ |
+ |
5 |
|
A. oryzae (Ahlbarg) Cohn |
+ |
+ |
+ |
+ |
+ |
5 |
|
A. parasiticus Speare |
+ |
+ |
- |
+ |
+ |
4 |
|
A. terreus Thom |
+ |
+ |
+ |
+ |
+ |
5 |
|
A. Versiculor (Vuill) Tiraboschi |
+ |
- |
+ |
+ |
- |
3 |
|
Botryodiplodia theobromae Sacc |
- |
+ |
+ |
+ |
- |
3 |
|
Botrytis cinerea Pers |
+ |
- |
+ |
- |
+ |
3 |
|
Fusarium culmorum (W.G.) Saac |
+ |
- |
- |
+ |
+ |
3 |
|
F. oxysporum Schlecht |
- |
+ |
+ |
- |
+ |
3 |
|
F. poae (Peck) Wollenw |
+ |
- |
- |
+ |
- |
2 |
|
F. sporotrichoides Sherb |
+ |
- |
+ |
+ |
- |
3 |
|
Helminthosporium velutinum link |
- |
+ |
+ |
- |
+ |
3 |
|
Penicillium paraherquei Abe ex.G.Smith |
- |
- |
- |
+ |
+ |
2 |
|
Scytalidium thermophilium Austwick |
+ |
- |
- |
+ |
- |
2 |
|
Mortierella isabellina Dudem |
+ |
+ |
- |
+ |
- |
3 |
|
M. nana Linnem |
- |
+ |
+ |
- |
- |
2 |
|
Torula herbaram (Pers) Link |
- |
+ |
+ |
- |
+ |
3 |
|
Phycomycetes |
||||||
|
Mucor pusillus Lindt |
+ |
+ |
+ |
+ |
- |
4 |
|
Rhizopus oligosporus Ehrenb ex. Corda |
- |
- |
+ |
- |
+ |
2 |
|
Yeasts |
||||||
|
Candida albicans Berkhout |
+ |
+ |
+ |
- |
+ |
4 |
|
Rhodotorula sp. |
+ |
+ |
+ |
+ |
+ |
5 |
|
Saccharomyces cerevisiae Hansen |
+ |
+ |
+ |
+ |
+ |
5 |
|
Al |
17 |
21 |
21 |
20 |
19 |
100 |
Table 1 Species of fungi isolated from the decaying sweet potato root tubers
+ Present , - Absent
CIP =CIP 4400168, EX = Ex-Igbariam, TAN =Tanzania TIS81 = TIS 8164, TIS87 = TIS 87/0087.
The fungal isolates included: 3 Ascomycetes, 22 Hyphomycetes and 2 Phycomycetes. The isolates also included 2 species of thermophiles (Mucor pusillus lindt and Scytalidium thermophilum Austwick), 4 species of thermotolerants and 24 mesophiles. The genus Aspergillus had the highest number of species (10), followed by Fusarium (4), Eurotium (2) and Mortierella (2) The rest of the genera isolated had at least one species each.
Three yeast species: Candida albicans Berkhout, Rhodotorula species and Saccharomyces cerevisiae Hansen were also isolated in the decaying root tubers, reinforcing the presence of sugar in the stored produce (Table 1). The yeasts were found to have fermented and assimilated different sugars (Table 2). The yeast isolates Rhodotorula species and Saccharomyces cerevisiae contain species that have fermentative abilities that could ferment the sugar component of the root tubers under favourable conditions to further initiate the decay process.9
|
Gram staining |
Germ Tube Test |
India Ink Test |
Fermentation test |
Sugar assimilation test |
Ureae Test |
Growth at 250C |
Growth at 370C |
Growth at 450C |
|
Yeast |
||||||||
|
Gal |
Glu |
Lac |
Matt |
Suc |
Gal |
Glu |
Lac |
Matt |
Suc |
|||||||||
|
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
+ |
+ |
- |
+ |
+ |
- |
+ |
+ |
- |
|
Candida aIbicans (Berkhout) |
|
+ |
- |
- |
- |
- |
- |
- |
- |
- |
+ |
- |
- |
- |
- |
+ |
+ |
- |
|
Rhodotorula sp. |
|
+ |
- |
- |
+v |
+ |
- |
+v |
+v |
-v |
+ |
- |
+v |
+v |
- |
+ |
+ |
- |
|
Saccharomyces cerevisiae (Hansen) |
Table 2 The biochemical features of the yeast isolates
V =Variable reaction none exhibited urease activity.
The experiment on amylase production by some of the fungal isolates from the decayed root tubers revealed that A. niger, A. oryzae, A. terreus, E. nidulans and Mucor pusillus were amylolytic and therefore had the ability to hydrolyse the starch component of the root tubers to glucose for other fungi to utilize. Hence these amylolytic species might have promoted the proliferation of the other species identified more on the surfaces of the stored root tubers than the in-depth layers.
The physico-chemical determinations showed that the freshly harvested Tanzania sweet potato root tuber had a surface (peel) moisture content (MC) of 49.60% and in-depth (5th layer) moisture content (MC) of 52.55% (Figure 1); however, the same cultivar was found to have 31.52% surface MC and 45.36% in-depth (MC) after 8 weeks of storage (Figure 5) The fresh CIP 4400168 root tubers which followed Tanzania in the same tread of MC dispersal ranged between 48.21% for the surface (peel) and 52.45% in-depth (5th layer) MC but had 39.41% surface MC and 46,45% in-depth (5th layer) MC after 8 weeks of storage in the barn. The root tuber surface (peel) and the layers towards it were lower in MC in comparison to the in-depth layers of its circumference. This indicated that MC migration was directed towards the tuber surfaces (peels) which had lower values of the MC than the in-depth layers with higher values.
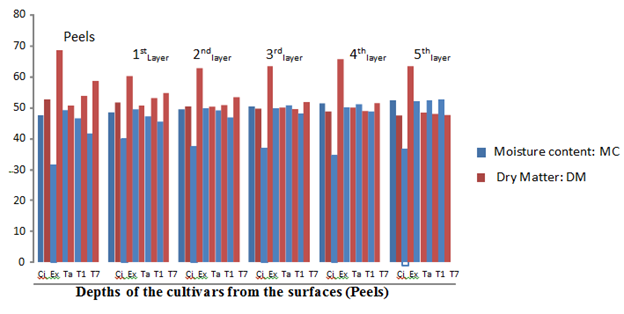
Figure 1 The percentage moisture content and dry matter of the root tubers at different depths from the sources before storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =IS8164 and T2 =IS87/0087
Similar MC was recorded for the other cultivars fresh root tubers (before storage) and after 2, 4, 6 and 8 weeks of storage in the barn (Figures 2, 3, 4 and 5). The MC loss which became evident after 2 weeks of storage of the root tubers in the barn caused the dry matter to become concentrated with glucose especially at the surfaces (peels) than the in-depths layers of the root tubers, hence more fungal species were isolated at the peels. It can be observed in Figures 1-5 that when the root tubers MC dwindled during storage the dry matter water activity (ɑw) was lowered and the sugar content increased and became most suitable for fungal utilization and proliferation resulting in the root tuber deterioration.
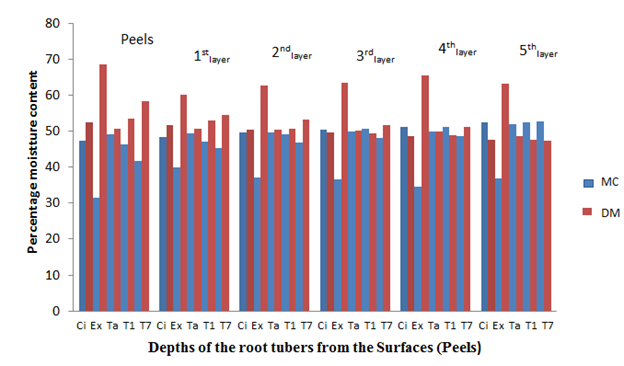
Figure 2 The percentage moisture content and dry matter of the root tubers at different depths from the surfaces after 2 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
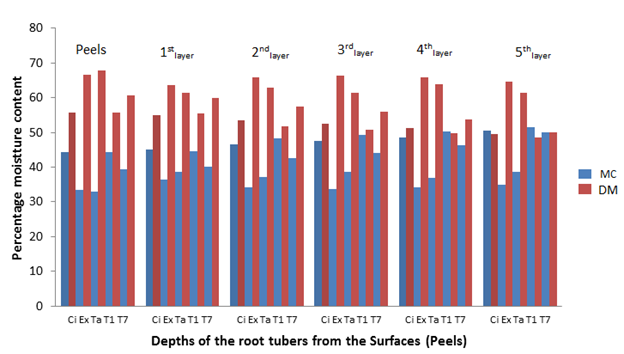
Figure 3 The percentage moisture content and dry matter of the root tubers at different depths from the surfaces after 4 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
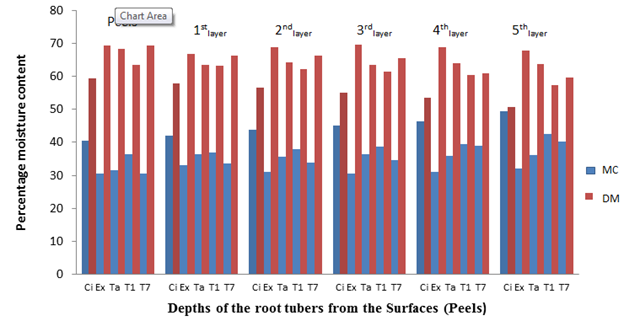
Figure 4 The percentage moisture content and dry matter of the root tubers at different depths from the surfaces after 6 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
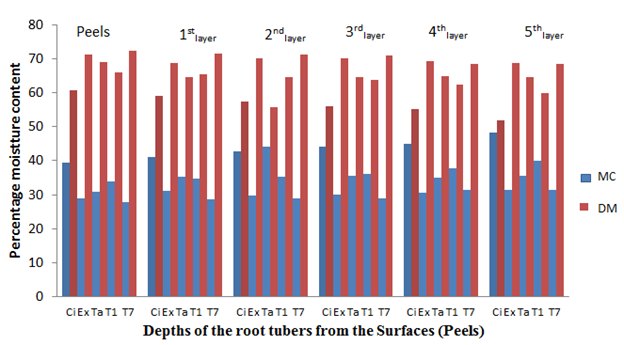
Figure 5 The percentage moisture content and dry matter of the root tubers at different depths from the surfaces after 8 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
The fresh root tuber of Ex-Igbariam had the highest surface glucose content of 11.98% and an in-depth glucose content of 0.21% as compared to CIP 4400168 which had 7.51% surface glucose content and in-depth glucose content of 6.41% (Figure 6). However, after 8 weeks of storage, Ex-Igbarium was found to have 14.71% surface glucose content while CIP 4400168 glucose content had increased to 9.67% (Figure 10). Similar tread in terms of greater surface glucose were recorded for the other cultivars after 2, 4 and 6 weeks of storage in the barn (Figures 7, 8 and 9). The biochemical analyses on the harvested root tubers showed that glucose (a reducing sugar) was dispersed within the circumference of the stored root tubers in different concentrations (Figures 6, 7. 8, 9 and 10). Most of the peel isolates actually colonized the root tubers due to the abundance of glucose and eventually led to their deterioration.8
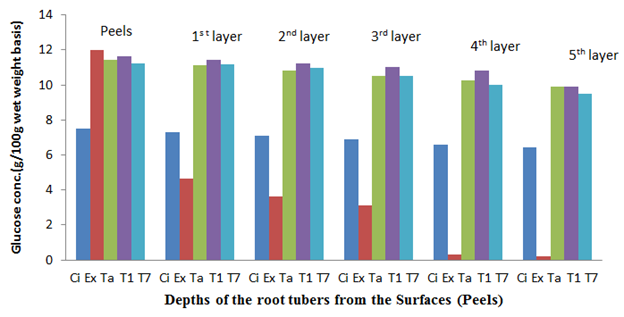
Figure 6 The glucose content of the sweet potato root tubers at different depths from the surfaces (Peels) before storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
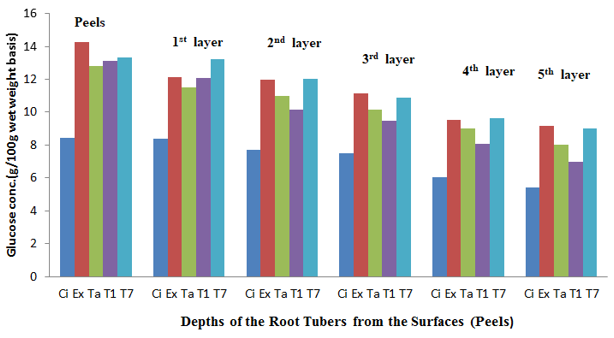
Figure 7 The glucose content of the sweet potato root tubers at different depths from the surfaces (Peels) after 2 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
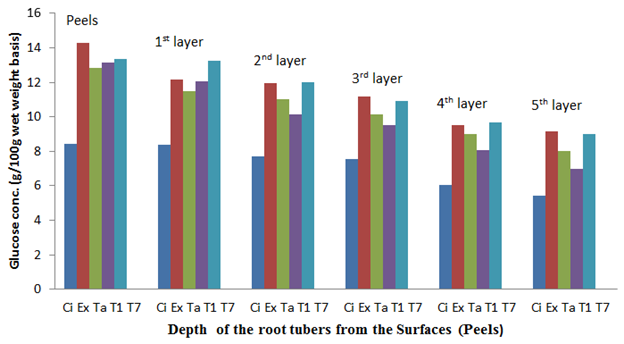
Figure 8 The glucose content of the sweet potato root tubers at different depths from the surfaces (Peels) after 4 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
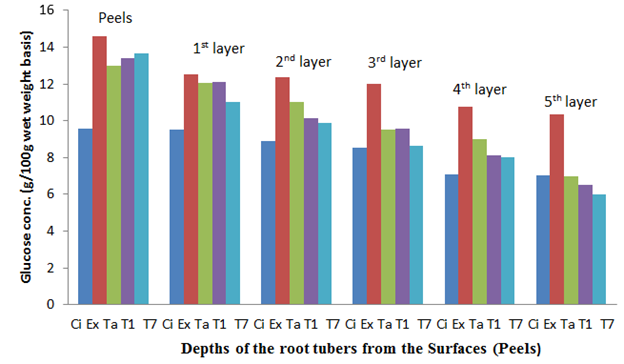
Figure 9 The glucose content of the sweet potato root tubers at differet depths from the surfaces (Peels) after 6 weeks of storage.
Key: Ci= CIP 4400168, Ex= Ex-Igbariam, Ta= Tanzania, T1 =TIS8164 and T2 =TIS87/0087
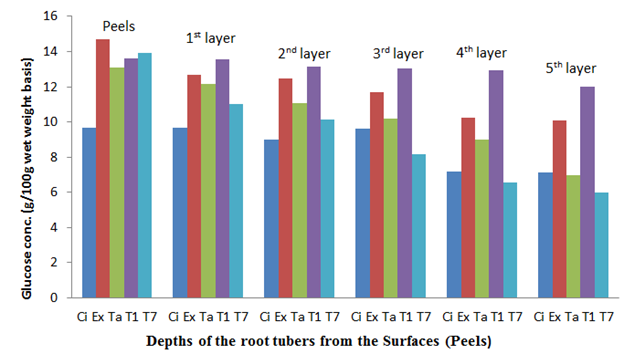
Figure 10 The glucose content of the sweet potato root tubers at different depths from the surfaces (Peels) after 8 weeks of storage.
Fungal penetration of the decaying sweet potato root tubers
The results obtained from the investigation on depth of fungal penetration of the decayed sweet potato root tubers showed that the highest number of fungal colonisers was on the surface layer of the decayed tubers which incidentally had the highest concentration of glucose. Glucose is the simplest form of sugar which does not need further break-down before it enters the glycolytic pathway in the respiratory process. The high concentration of sugar on the surface of the tubers therefore provided the fungal colonizers the necessary energy they required for growth, development, proliferation and eventually the deterioration of the root tuber.
The number of fungal species decreased from the surface of the tubers to the central portions of the decaying root tubers. Seven species of Phycomycetes were isolated from the first 4 layers of the decayed tubers. The Phycomycetes therefore had a deeper penetration of the tubers than the rest of the fungal colonisers. M. pusillus was isolated from all the layers. The occurrences of the Phycomycetes in all the layers seem to be tied up with sugar concentration. The Phycomycetes are known as sugar fungi and it is therefore not surprising that the organisms had more penetration. These fungi are found in carbohydrate substrates that have substantial sugar contents.25 A oryzae which is a good amylase producer and which is used a lot in fermentation processes had 100% in terms of the decayed tubers penetration
Occurrence of the fungal colonisers in different layers of the decayed sweet potato root tubers
The depth of penetration of the decayed sweet potato root tubers by the fungal colonisers revealed that the highest number of fungal isolates (33) was made from the first layer (1) that contained the peels. The second highest number of fungal isolates (21) was made from the second layer (2) while the third highest number of fungal isolates (17) was made from the third layer (3) from the surface. The least number (2) of fungal isolates was made from the central layer 7. The details of the fungal isolation experiment in terms of fungal hyphae penetration of the decayed sweet potato root tubers is presented in Table 3.
Fugal isolates |
Occurrence in different depths from the surface |
|||||||
|
1 |
2 |
3 |
4 |
5 |
6 |
7 |
Total |
|
|
Ascomycetes |
|
|
|
|
|
|
|
|
|
Emericella nidulans (Eidem) Vuill |
+ |
+ |
- |
- |
- |
- |
- |
2 |
|
Eurotium amstelodami Mangi |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
E. harbariorum (Wiggers) Link |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Hyphomycetes |
|
|
|
|
|
|
|
|
|
Asperqillus candidus Desm |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
A. clavatus Desm |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
A. carneus Blochwitz |
+ |
+ |
- |
- |
- |
- |
- |
2 |
|
A. flavus Link ex. Gray |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
A. fumigatus Fres |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
A. niger van Tieghem |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
A. oryzae (Ahlburg) Cohn |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
7 |
|
A. parasiticus Speare |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
A. terreus Thom |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
A. versicolor (Vuill) Tiraboschi |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Botryodiplodia theobromae Saac |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Botrytis cinerea Pers |
+ |
+ |
- |
- |
- |
- |
- |
2 |
|
Fusarium calmorum (W.G.) Saac |
+ |
+ |
- |
- |
- |
- |
- |
2 |
|
F. oxysporum Schlecht |
+ |
+ |
+ |
|
- |
- |
- |
3 |
|
F. poae (Peck) Wollenw |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
F. sporotrichoides Sherb |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
Helminthosporium velutinum Link |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
Penicillium paraherquei Abe ex. G. Smith |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Scytalidum thermophilum Austwick |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Torula herbarum (Pers) Lick |
+ |
- |
- |
- |
- |
- |
- |
1 |
|
Phycomycetes |
|
|
|
|
|
|
|
|
|
Mortierella isabellina Oudem |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
M. nana Linnem |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
M. ramanniana (Moller) Linnem |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
M. vinacea Dixor Stewart |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
Mucor pusillus Lindt |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
7 |
|
Rhizopus oligosporus Ehrenb ex. Corda |
+ |
+ |
+ |
+ |
- |
- |
- |
4 |
|
Syncephalastrum racemosus Cohn |
+ |
+ |
+ |
+ |
+ |
+ |
- |
6 |
|
Yeasts |
|
|
|
|
|
|
|
|
|
Candida albicans Berkhout |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
Saccharomyces cerevisiae Hansen |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
Rhodotorula sp |
+ |
+ |
+ |
- |
- |
- |
- |
3 |
|
Total |
33 |
21 |
17 |
9 |
3 |
3 |
2 |
88 |
Table 3 Occurrence of fungal colonisers at different layers of the decayed root tubers
+ Present – Absent
CIP =CIP 4400168, EX = Ex-Igbariam, TAN =Tanzania TIS81 = TIS 8164, TIS87 = TIS 87/0087.
The occurrences of most of the isolates in the outer layers and the peels specifically seem to be tied up with the glucose concentration. The results revealed that some of the biodeteriogens in different layers of the decayed root tubers were not mere surface contaminants but had an in-depth penetration of the root tubers. The deep penetrators were aided by amylases they produce that could hydrolyse the starch component of the root tuber to glucose. The inherent amylases especially those produced during sprouting also hydrolysed the starch component to glucose.26 As the moisture content in the tuber migrates exteriorly, the excess glucose is carried a long side it and deposited at the surface (peel) of the root tuber, accounting for a higher population of fungal biodeteriogens at this layer and those close to it. These activities aided the tubers colonisation by the biodeteriogens which brought about more hydrolysis of their starchy component and the eventual deterioration of their circumference into different decayed rots produced by the different fungal species.
It is small wonder then why species of yeasts were also isolated from the decaying tubers. The sugar constituent of the tubers could be what was responsible for the yeasts colonisation of the storage root tubers.27 The yeasts are popular for enzyme production which is employed to ferment the sugars of the root tubers to further initiate the decay process.
Some of the fungal isolate were tested for their abilities to produce amylase needed for the hydrolysis of the starch component of the root tubers carbohydrate. A. niger, A. oryzae, A. terreus, E. nidulans and Mucor pusillus were amylolytic and therefore had the ability to hydrolyse the starch component of the root tubers to glucose for other fungi to utilize. Such could lead to the eventual decay of the stored sweet potato root tubers.
A proof that there was higher glucose concentration at the surface layer of the tubers was the kind of results obtained during the biochemical analyses of glucose concentrations in different layers of the root tubers (Figures 6–10). Also as the root tubers became more dehydrated, they become sweeter. This shows that there is an enzymatic activity in the tubers which breakdown the starch component of the tubers to glucose. Such enzymatic activity could stem from a combined amylases production from the colonising fungi and the inherent amylolytic activity in the root tubers induced by sprouting.28
This study has shown that the deteriorative and degradative activities of fungal biodeteriogens during storage of sweet potato root tubers are greatly influenced by moisture content migration towards the peels which mobilised and concentrated glucose towards it, encouraging the proliferation and growth of the biodeteriogens. Their presences at the peels reduce drastically the shelf-life of the farm produce/market commodity popular for its food value on the Jos Plateau. To increase extensively the shelf-life of the harvested root tubers, plant extracts are being experimented to completely control enzymatic browning of the farm produce to formulate secondary products such as sweet potato starch that could be utilised in food, pharmaceutical and textile industries; flour employed to formulate bread, biscuit, cake and chin-chin (a local pastry).
The next line of duty in the study of the stored root tuber is to examine the effects of moisture content immigration on the water activity aw of the dry matter content in furtherance of the study in the keeping quality of the produce. Also to determine the favourable and the non-favourable environmental parameters of the in-door storage barn on the keeping quality of the stored root tubers.
The authors declare that there are no conflicts of interest.

©2024 Sila, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.