Journal of
eISSN: 2469 - 2786


Research Article Volume 7 Issue 1
1Shardabai Pawar Mahila Mahavidyalaya, India
2Malegaon Sheti Farm, Agricultural Development Trust Baramati, India
Correspondence: Vitthalrao B Khyade, Malegaon Sheti Farm, Agricultural Development Trust Baramati, India
Received: June 06, 2018 | Published: February 21, 2019
Citation: Seema KD, Priti MG, Shubhangi SP, et al. The influence of infection of Beauveria bassiana (Bals) Vuill, a fungal species (Family: Clavicipitaceae) on quality of the cocoons of spinned by the larval instars of Bombyx mori (L) (Race: PMx CSR2). J Bacteriol Mycol Open Access. 2019;7(1):14-18. DOI: 10.15406/jbmoa.2019.07.00234
The Beauveria bassiana (Bals) Vuill is an entomopathogenic fungal species belong to the family: Clavicipitaceae. The entomopathogenic fungal parasites are causing the muscardine disease in larval instars of silkworm, Bombyx mori (L). The muscardine disease in the larval instars of silkworm is caused by fungus, Beauveria bassiana (Bals) Vuill. The Beauveria bassiana (Bals) Vuill is one of the most destructive pathogen of silkworm Bombyx mori L and it is common in all sericulture zones of the world. The fungal disease is common during winter and rainy seasons. White muscardine was the first disease described in insects caused by a microorganism. This fungus was named eventually Beauveria bassiana in honor of Agostino Bassi, sometimes called de Lodi (25 September 1773–8 February 1856), Italian entomologist, botanist and bacteriologist recognized as the “Father of Insect Pathology”. Beauveria bassianainfection influenced the growth and development of silkworm larvae and ultimately the economical cocoon characters like matured larval weight, cocoon weight, shell weight, shell percentage, filament length, non-breakable filament length, number of breaks and denier. Significant reduction of matured larval weight (2.08g), cocoon weight (0.72g), shell weight (0.09g), shell ratio (12.80%), filament length (478.9m), non-breakable filament length (120.8m) and more number of breaks (4.1) and higher denier (2.62d) was recorded in the experimental silkworm compared to control.
Keywords: Fungal diseases, Beauveria bassiana, Bombyx mori, symptom logical, cocoon yield parameters
The Beauveria Bassiana (Bals.) Vuill is a well recognized entomopathogenic fungal species belong to the family: Clavicipitaceae. It is acting as a parasite on species of arthropods. It is causing white muscardine disease the white muscardine poses a major threat to silk cocoon production during rainy and winter seasons. The rainy and winter seasons are congenial for the spread of white muscardine disease in larval instars of silkworm, Bombyx mori (L). It is therefore important to resort to appropriate management practices to prevent the outbreak of the disease at the field level. Obviously, the success of sericulture industry primarily depends on the successful harvest of cocoon crops. The major problem of sericulture in a tropical country like India is the high incidence of diseases. The major diseases affecting mulberry silkworm are muscardine (fungal disease), flacherie (bacterial diseases) grasserie (viral diseases) and pebrine (protozoan disease). Silkworm crop loss occurs in all the silkworm growing areas of the world, but the type of severity varies. They differ from region to region, crop to crop and even from farmer to farmer.
Leaving aside minor variations, it has been found that crop loss is generally more in the tropics than in the temperate regions. The magnitude of the disease damage is on the higher side in India. It is a general observation that out of 5-6 crops per year, two are usually lost due to diseases and other reasons and even the successful once are partially lost. Thus the frequent outbreak of diseases is one of the main handicaps for the progress of sericulture industry. The reasons attributed, are poor hygienic conditions and continuous silkworm rearing all round the year, the climatic conditions that favour faster multiplication of disease causing germs. The accumulated germs under favourable conditions become active and cause the outbreak of diseases. Beauveria bassiana (Bals) Vuill is one of the most destructive fungal pathogen of silkworm Bombyx mori L causing white muscardine disease, which is common in all sericulture zones of the world. The fungal disease is common during winter and rainy seasons. White muscardine was the first disease described in insects caused by a microorganism.1 Demonstrated the contagious nature of the disease and identified the cause as a fungus and suggested measures to control the disease.2 The fungus was named after Agostino Bassi Vuillemin in his honour as Betrytis bassiana by Balsamo and created a genus Beauveria bassiana (Balsamo) Vuillemin. It was a first microorganism to be recognized as a disease causing agent.1 this disease wiped out the entire sericulture industry in Italy and France during 1920 1925. In India 10 40percent of loss has been accounted for white muscardine in total loss due to diseases.3‒5 In the year 2005, heavy rain in major silk producing states of Karnataka, Tamil Nadu and Andhra Pradesh has led to a widespread outbreak of muscardine among the silkworm cocoon crops, threatening the silk output in the country. In this backdrop, the study was carried out to examine the symptological changes in Bombyx mori during the progress of the disease and its influence on economical characters of the cocoons.
The silkworm race PM x CSR2 was selected for the study and the silkworms were reared under optimum conditions with meticulous coordination of many activities such as maintenance of mulberry garden, preparation and disinfection of the rearing room and appliances, procurement and handling of silkworm eggs and incubation, young and late age silkworm rearing, general hygiene, moulting care, mounting, spinning, harvesting of cocoons etc.6 The larvae were processed further as per the guidelines enlisted by.7 On the 1st day of the fifth instar, the larvae were inoculated by dipping in sublethal concentration of fungal conidia spore suspension (2.15x 106 conidia spores/ml @ 50ml/100 worms for 45Sec) and larvae treated with double distilled water were used as control. From the day of inoculation to the end of the 5th instar the silkworms were kept under continuous surveillance to examine the symptom logical changes during the development of fungal pathogen Beauveria bassiana and taken photographs. By the end of the fifth instar the silkworm showed the symptoms of spinning i.e., cessation of feed, body shrinkage, translucent light yellow colour skin etc. These larvae are known as matured worms, the weight of the worms was recorded. These worms were transferred to mountages to spin the cocoons. After completion of the spinning process, the cocoons were harvested on the 6th day of mounting and then these cocoons were taken for the assessment of various economic parameters viz., cocoon weight, shell weight, shell ratio, filament length, non-breakable filament length, number of breaks and denier. The qualitative and quantitative parameters of cocoons were determined by following the methods as given by.8
Matured larval weight
The Quantitative profile analysis of fifth instar larval silk worms was studied by weighing and measuring the worms during 1-11 days of fifth instar. For this purpose, ten larvae from every replication were randomly selected and their individual weight was recorded. This character indicates the healthy and robust disposition of the larvae.
Cocoon weight
The silk cocoon weight which is important trait in sericulture because cocoon weight has moderate heritability and is actually easy to quantify researchers select original populations based on this trait. Cocoon weight indicates the approximate quantity of raw silk that could be reeled from the cocoons.9 In the present experiment a sample is drawn from each replication comprising around 10cocoons. The sample drawn represents the entire quality of each replication. Individual cocoon weight was taken from each sample of 10cocoons and mean cocoon weight was calculated. The weights were taken in gram units.
Shell weight
Cocoon shell weight is economic trait represents the total quantity of silk in a cocoon. Average single shell weight was calculated from 10shells used for the assessment of cocoon weight.
Shell ratio
Cocoon shell denotes the total amount of silk available in a single cocoon and is expressed in percentage. It is calculated by dividing the shell weight reading by cocoon weight reading. Quotient thus obtained was multiplied by hundred to obtain the shell ratio or shell percentage in entire cocoon.
Filament length
Silk filament is the total length of silk filament, unwound from a single cocoon measured in meters. In the present experiment a sample is drawn from each replication comprising around 10cocoons. Ten cocoons were cooked and reeled on an eprouvette with a circumference of 1.125m and the mean value of filament length in meters was calculated as per the standard formula.8
Non-breakable filament length and Number of Breaks
It is the average length of filament that can be unwound from the cocoons without a break. Non-breakable filament length was calculated by using the formula as given by,8 and the numbers of breaks were recorded.
Denier
Denier scale is the weight in grams of 9,000 meters of the filament. Denier represents the size of the yarn. It was calculated using the formula as given by.8
Variation in the denier of the cocoon silk filament will ultimately determine the uniformity and quality of raw silk yarn reeled.
Statistical analysis
For the consistency in the results, all the attempts were revised three times. The data was collected. All the experimental data recorded from the three replicates have been subjected to statistical analysis by following T-test.
The results on Influence of Infection of Beauveria Bassiana (Bals) Vuill, A fungal species (Family: Clavicipitaceae) on quality of the cocoons of spinned by the larval instars of Bombyx mori (L) (Race: PM x CSR2) are summarized in Table 1 and explained away in parameter wise (Symptom logical Changes and Economical cocoon parameters).
Parameters |
Matured |
Cocoon |
Shell |
Shell ratio |
Filament |
Non- |
Number |
Denier |
Control |
2.58 (±0.0) |
1.44 (±0.06) |
0.24 (±0.3) |
16.43 (±0.12) |
671.6 (±18.33) |
640.6 (± 14.08) |
0.2 (± 0.42) |
2.20 (±0.12) |
Diseased (Inculcated) |
2.08 (±0.07 ) |
0.72 (±0.17) |
0.09 (±0.01) |
12.80 (±0.10) |
478.9 (±15.74) |
120.8 (± 22.42) |
4.1 (± 2.42) |
2.62 (±0.07) |
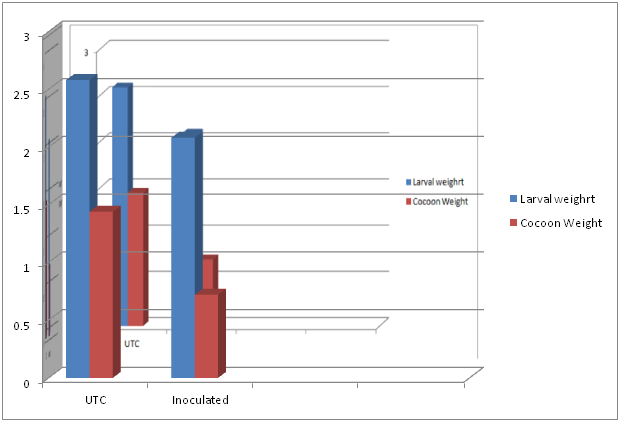
Table 1 Changes in economical cocoon parameters of silkworm Bombyx mori L. inoculated with fungal pathogen Beauveria bassiana (Bals.) Vuill with reference to control.
Symptomological changes
No conspicuous symptoms were noticed immediately after inoculation of fungal pathogen Beauveria bassiana. But gradually an infected silkworm became inactive, sluggish, stopped the feed and remained underneath the mulberry leaves. After 48hours, the infected worms started to vomit digestive juice and later, the worms gradually became stiff and the movement of the worms was very much restricted and colour of the body changed to brown colour with oily specks. Initially the oily specks are in small in size with the advancement of the age, the size and number of the oil specks was enhanced (Figure 1) (Figure 2). Then the silkworm body became soft, pliable and later stiff and hard. Nearly on 7th or 8th day of the infection white efflorescence noticed near intersegment region, spiracles, and then complete body was covered with the white mycelia and finally conidia developed on the body Figure 3. The mummified cadaver became brittle and breaks into pieces when dropped from a certain height. Infected worms fail to spin the cocoons, but those which spin form flimsy cocoons Figure 4. Cocoons formed by these infected worms were smaller and lighter in weight and the worms not emerged as moths.
Economical cocoon parameters
The overt changes observed in the economical traits of cocoon in Beauveria bassiana infected silkworm with reference to control are shown in Table1 and Figure 5 & Figure 6. The results of the present study clearly indicated that Beauveria bassiana infestation influences the growth and development of silkworm and the economical cocoon parameters. Significant reduction of matured larval weight (2.08g), cocoon weight (0.72g), shell weight (0.09g), shell ratio (12.8%), filament length (478.9m), non-breakable filament length (120.8m) and more number of breaks (4.1) and higher denier (2.62d) was recorded in the experimental silkworm larvae. In contrast, to the experimental larvae significantly higher values were recorded in the majority of the economical characters such as matured larval weight (2.58g), cocoon weight (1.44g), shell weight (0.24g), shell ratio (16.43%), filament length (671.6m), non-breakable filament length (640.6m) and less number of breaks (0.2) and lower denier (2.2d) in control.
Appropriate matured larval weight is an indicator to measure the health of silkworm and in turn to obtain good quality of cocoon. Cocoon weight is an important commercial character used to determine the amount of raw silk that can be obtained. The cocoon price is determined based on the weight of the cocoon. The shell weight is more important than the cocoon weight since it is the shell that yields the silk for reeling. Thus, higher the weight of the shell, greater will be the silk yield. Shell percentage fairly indicates the quantity of raw silk that can be reeled from the cocoons and also helps in estimating renditta and thereby fixing a proper price for the cocoons. The silk filament length indicates the reelable length of silk filament from a cocoon. Reeling performance is better with cocoons having a larger filament length, which reduces the number of feeding ends. This results in increase of production, quality and also raw silk percentage of the cocoon. Generally, a longer non-breakable filament with less number of breaks, higher is the reelability. This indicates that higher the reliability percentage higher is the raw silk quality.
The fineness of cocoon filament is expressed by size i.e. denier.8,9 Reduction in the matured larval weight may be due to the consequence of fungal infection that leads to the decrease in food consumption, digestion, relative consumption rate, efficiency of conversion of ingested food in fifth instar of Bombyx mori infected with Beauveria bassiana.10 analyzed the fungus growth and proliferation in silkworm body after 12 to 24h post- inoculation of the B. bassiana conidium. The healthy growth and development of the silkworm is directly related to economical cocoon traits. It is well supported by.11 She noticed poor cocoon characters from pebrine infected tropical tasar silkworm (Figure 7) (Figure 8).
Antheraea mylitta The reduction in economical cocoon characters could be attributed to loss of appetite, lethargic conditions and physiological stress induced by the fungal pathogen Beauveria bassiana in 5th instar silkworm larvae.12 Observed the reduction of economical parameters due to Beauveria bassiana infection and attributed that it may be due to the decline in the synthesis of silk proteins and the direct or indirect effect of Beauveria bassiana on the growth and development of silk gland of the silkworm Bombyx mori. More number of breakages and higher denier were noticed in experimental cocoons with reference to healthy ones. It may be assumed that the physiological and biochemical stress induced by a fungal pathogen caused to exude uneven amounts of silk fluid in lumps.13 Have reported the decrease in shell weight in Antheraea mylitta larvae infected.14,15 studied on the parasitization of fifth instar larvae of Antheraea mylitta by Uzifly have reported the decrease in cocoon weight and shell weight in the infected larvae.
Silkworm Bombyx mori is completely domesticated economic insect and Beauveria bassiana is an aggressive parasite. Therefore, the disease in silkworm results in a drastic reduction in the growth and development and qualitative and quantitative cocoon yield parameters. This ultimately has a direct effect on the sericulture community of the country due to reduced returns, in turn affecting the economy of the country, since silk as the end product of sericulture industry is a good source of foreign exchange for the country. Therefore, it is the prime responsibility of sericulture scientists and sericulture farmers to explore many more strategies to prevent/control the occurrence of the disease to enhance the qualitative and quantitative parameters of cocoon crops.
None.
The author declares there is no conflicts of interest.

©2019 Seema, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.