Journal of
eISSN: 2469 - 2786


Short Communication Volume 12 Issue 3
Associate Professor, Amity Institute of Biotechnology, Amity University, India
Correspondence: Dr. Kirti Rani, Associate Professor, Amity Institute of Biotechnology, Amity University Uttar Pradesh, Noida, Sec-125, Gautam Buddha Nagar, Noida-201313 (UP), India
Received: September 24, 2024 | Published: October 22, 2024
Citation: Rani K. Succinct review on emergent crisis of antibiotics resistant (MDR) bacteria: impact on human health worldwide. J Bacteriol Mycol Open Access. 2024;12(3):94-96. DOI: 10.15406/jbmoa.2024.12.00380
Multidrug resistance (MDR) has become an alarming global health menace due to either overuse or abuse of antibiotics. It has happened because of lack of proper diagnostic testing and absence of general medical awareness including overuse of antibiotics in livestock and animal husbandry. Indeed, increased MDR phenomenon is led to increase in global mortality and morbidity because of diseases treatment failures that impacted global healthcare costs. It is done due to inappropriate continual use of antibiotics in human therapies, in animal husbandry and aquaculture farming practices that resulted in increase the incidence of pathogenic bacteria becoming resistant to multiple drugs. Multidrug resistant (MDR) bacteria are very common to become prevalent causes of community-acquired infections that associated with increased morbidity, mortality and healthcare costs. Colonisation of foreign patients by MDR bacteria is found to major health concern as predisposing factors in hospitals. During hospitalization and ICU treatment of critically ill foreign patients including travellers/tourists are found to having increased risk of acquiring MDR bacteria when hospitalized abroad. Hence, all these multiple factors led to fuelling in antibiotic resistance of bacteria during pandemic crisis and causing lethal effect in cancer patients due to treatment. Antibiotic resistant pathogens to protect world human population from this global health threat. So, this stipulated review is highlighted the insight of various notable aspects of fuelling of antibiotic resistance in bacteria including their possible modes of actions and ingrained impacts on human health. Henceforth, it is the peak era to explore new sustainable therapeutic alternatives to curb increase in global MDR crisis.
Keywords: multidrug resistant bacteria, antimicrobial drugs, antibiotic susceptibility, foreign transmission risks, antibacterial resistance.
Nowadays, multidrug resistance (MDR) is a globally recognized health concern that made therapeutic drugs ineffective against various treatable diseases like tuberculosis, malaria, urinary tract infection, gonorrhea and pneumonia when most of MDR bacterial strain became superbugs (Figure 1).1–3
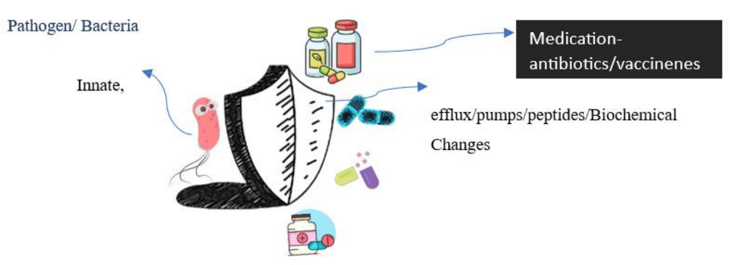
Figure 1 Pictorial simplified portrayal of multidrug resistant bacteria.1–3
Multi-drug resistant (MDR) bacteria are found to rise due to prolonged hospital stay, ineffective treatment failure that led to increase morbidity and increased health burden globally.4–7 Hitherto, it is need to prevent MDR bacteria transmission during hospitalization to keep suspected high risk patients in isolation following proper identification and preventive clinical policies by adopting more potent alternative of safe doses of antimicrobials.8–10 MDR bacteria are found to cause many communicable diseases that are more prone to spread fast in pandemic. It became a cut throat medical challenge in those countries where medical services are more advanced and where various foreign medical travelers and tourists are used to visiting very frequently. Hence, WHO has been classified notable multidrug-resistant bacteria as "priority pathogens" or "ESKAPE pathogens," e.g. Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species. These superbugs are reported to become difficult to kill due to having unique structural cell wall compositions including their respective increased density of drug efflux pumps.11
All Bacteria are found to acquire antibiotics resistance by well depicted cellular mechanisms like drug inactivation process carried out due to β-lactamases group of enzymes that hydrolyse drugs as part of the inactivation process. Other reported cellular route is drug uptake reduction which carried out by lowering of bacterial cell permeability as an example: Pseudomonas aeruginosa found to consist monomeric 8-stranded β-barrel with a C-terminal periplasmic domain. It is observed that a rare oligomer form has large open β-barrels which belong to diffusion porin OmpF.12,13 Another notable route of cellular mechanism is reported drug efflux take place outside the bacterial cell membrane. It is led to the characterization of drugs efflux pumps in various emerging superbugs like methicillin-resistant Staphylococcus aureus (MRSA), Streptococcus pneumoniae, Clostridium difficile, Enterococcus spp., Listeria monocytogenes, Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, Stenotrophomonas maltophilia, Campylobacter jejuni, Pseudomonas aeruginosa, Neisseria gonorrhoeae, Vibrio cholerae and Salmonella spp.14–16
In last decade, many reported MDR bacterial strains are found to subject for studying their altered biochemical metabolic pathway which make them to escape their target site from binding to proposed antibiotics/drugs. This is led to decreases the affinity of antibiotic molecules while targeting against specific bacterial killing of bacterial strains that synthesize essential folic acid and nucleic acid from the para-amino benzoic acid (PABA) precursors. Hence, it is restricted the efficacy of antibiotics like sulphonamide that block PABA pathway, so that targeted bacteria became resistant against proposed antibiotics/ therapeutic drugs (Figure 2).17
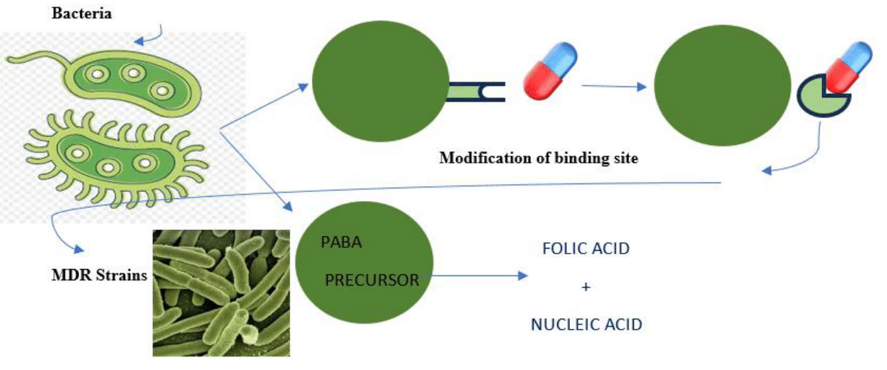
Figure 2 Pictorial delineation of defence mechanism of bacteria by modifying itself as MDR bacterial strain against proposed antibiotics.17
MDR bacterial pathogenic strains are found to have increased expression of active efflux pumps that in turn decreasing the drug permeability from the cell surface. This resulted in low susceptibility to antibiotics especially fluoro quinolones-based medications. Most of gram-negative bacteria are found to use this drug efflux mechanism whereas gram-positive bacteria lack this drug efflux mechanism because of not having lipopolysaccharides in their outer membrane. And, few of MDR gram-negative bacteria are also found to produce plasmid-mediated genes which are selectively bind with DNA gyrase or topoisomerase IV that make them protected from the quinolones-based antibiotics while given to patients during hospitalization as first line drug treatment.18
Medical tourism is found to play a major role in spreading multidrug resistant bacteria (MDR) globally. A previous clinical cohort study is carried between 2009-2014 in Hadassah Medical Center that is known referral center for medical tourists worldwide and also for Palestinian Authority residents.19–21 It was found that trade/travel tourists and patients from the Palestinian Authority suffered from high indexed rates of MDR positivity as MDR carriers when compared to Israeli patients than the resident population including antimicrobial resistance. Hospitalized foreign travellers are found at high risk of acquiring antimicrobial resistant bacteria in Europe report having MDRO carriage rates.21–24 MDRO based clinical investigation was done in Finland where MRR found more prevalent in foreign hospitalized patients. It is also found to be more strongly associated with travellers from the Indian subcontinent followed by Southeast Asia, Africa and South America due to lack of proper clinical guidelines for medical tourism raising bloodstream infection (BSI): a life-threat and increasing health care costs as well.25–28 The increasing burden of hospital-acquired BSI caused by Multidrug-resistant (MDR) pathogens are listed out in Table 1. Reported clinical studies was done to identity present bacterial etiology and predicting mortality in hospital acquired blood stream infections (BSI) in hospitalized patients and ICU admitted critical ill patients. Hence, MDR bacterial strains are found to be as potent independent predictor for calculating patients’ mortality who diagnosed with blood stream infections upon abroad hospitalization.29–31
This brief stipulated review is highlighted the various factors that lead to increase in incidence of MDR in exposed populations. Hospitalization of patients abroad including admitted in the ICU are found at higher risk of colonization and acquisition of MDR strain which must require proper screening and required isolation. So, increased medical tourism could lead to an increase MDR globally that lead to geographically dispersed outbreaks of various communicable diseases as well. Indiscriminate use of antibiotics and other overuse of antimicrobial agents have become mainstream cause of increasing MDR pathogens, “superbugs” in more suspected and exposed populations. Hence, we need to be more careful to control the spreading of MDR pathogenic bacterial strains in human population worldwide by adopting safe use of antibiotics and antimicrobial agents. National and International clinical governance must have to go ahead to analyzing and tracing these hazards and curtail ill effects of MDR on human health globally.
I would like to express my cordial appreciation to Amity University Uttar Pradesh, Noida (India).
The author declares that there are no conflicts of interest.

©2024 Rani. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.