Journal of
eISSN: 2469 - 2786


Research Article Volume 5 Issue 1
Microbial Biodiversity Directorate, Ethiopian Biodiversity Institute, Ethiopia
Correspondence: Birhanu Gizaw, Microbial Biodiversity Directorate, Ethiopian Biodiversity Institute, P.O. box 30726, Addis Ababa, Ethiopia, Tel 2.51912E+11
Received: June 05, 2017 | Published: June 30, 2017
Citation: Gizaw B, Tsegay Z, Tefera G, et al. Phosphate solubilizing yeast isolated and characterized from teff rhizosphere soil collected from gojam; Ethiopia. J Bacteriol Mycol Open Access. 2017;5(1):218-223. DOI: 10.15406/jbmoa.2017.05.00120
Phosphorous is an essential macronutrient for plant growth and development. About 95-99% present in soil insoluble form. Phosphate solubilizing microorganisms can increase soil phosphate solubility and availability. This study was aimed to identify and evaluate phosphate solubilizing yeast from Teff rhizosphere soil. Yeasts were identified using Biolog Micro station identification system. Yeast isolates were screened and transferred to biolog universal yeast agar media. Pure yeast cells were suspended in sterile water at 49±2 turbidity measured by biolog turbidimeter. 100μ- L transferred from each suspension into 96 wells of the biology yeast micro Plate tagged with different carbon source and incubated at 26°C for 24 to 72h and read by micro station at a single wavelength of 590nm, results were recorded and processed for identification by micro log3 software ver. 4.20.05. Above 0.5 similarities index value is acceptable species identification. Therefore biolog microstations identify nine yeast species with full species identity. The identified yeasts were tested for phosphate solubilization by the Pikovskaya’s agar (PVK) selective media. Nine yeast species were positive in phosphate solubilizing ability. Phichia norvegensis, Cryptococcus albidus var aerius, Candida etchellsii, Cryptococcus albidus var albidus, Rhodotrula aurantiacaA, Rhodotorula aurantiaca B, Cryptococcus luteolus, Cryptococcus albidus var diffluens, Cryptococcus terreus A.At 15days incubation their phosphate solubilizing index (PSI) ranges 1.72-3.35. Phichia norvegensis and Cryptococcus albidus var aerius were superior in phosphate solubilization 3.35 and 3.2 SPI respectively. Therefore these species can be candidated and exploited after further evaluation as bio fertilizers for teff productivity.
Keywords: biolog, microorganisms, microstation, phosphorus, rhizospher, soil, solubilization, teff
Improving soil fertility is one of the most common practices in agricultural productivity for all crops. Teff [Eragrostistef(Zucc.) Trotter] is the major indigenous cereal crop of Ethiopia, where it was originated and diversified. It is a highly demanded and a staple food grain for 60-65% of the Ethiopian people. In a country of over 80 million people, teff accounts for about 15% of all calories consumed in Ethiopia.1 More than 70-75% of Ethiopian highland soils are characterized by phosphorus deficiency.2 The deficiency is very severe in the acidic soils of the southern, southwestern and western regions. Areas Al3+ and Fe3+ high are totally incriminated with phosphorus fixation.3 Around 70% of Ethiopian vertisol have available phosphorus below 5 ppm, which is very low for supporting good teff growth and phosphorus fixation in vertisols is related more to calcium.4
Phosphorus is one of the major nutrients second to nitrogen required by plants for growth and productivity. It contributes remarkably to photosynthesis, sugar production, nucleic acid synthesis, and promotes N2 fixation in legume and energy production.5 It also increases the strength of cereal straw, promotes flower formation, fruit production, stimulates root development and also essential for seed formation, stalk and stem strength, maturity and production crop quality and resistance to plant diseases.6 A greater part of soil organic and inorganic phosphorus, approximately 95-99% is present in the form of insoluble phosphates that is bound by Al or Fe in acid soils, or Ca and Mg in alkaline soils which cannot be utilized by the plants easily.7,8
Declining soil fertility as a result of continuous cropping without replenishing soil nutrients continues application of phosphate fertilizer and soil erosions is the major factors that reducing production and productivity of the teff crop in Ethiopia. Higher grain yield of teff was recorded by applying inorganic fertilizers.9 However chemical fertilizers are neither easily available nor affordable for the majority of poor Ethiopian farmers and not environmentally friendly and also the recovery rate of Phosphate fertilizer by plants is only about 10 to 30%. The remaining 70 to 90% is accumulated in soil or in the form of immobile that is bound by Al or Fe in acid soils, or Ca and Mg in alkaline soils.7,8 Such economic considerations and phosphate existence in compound form necessitate for an alternative less expensive and environmentally friendly bio fertilizer improving yield and quality of teff grain. In Ethiopia, only few studies on teff root-associated microorganisms have been undertaken. The effect of phosphate solubilizing some fungus on growth and yield of teff was studied by Asfaw.10 Inoculation of teff by vascular arbuscular mycorrhazal (VAM) and plant growth promoting rhizobacteria (PGPR) give good result on teff productivity. So previous research works tell us using bio fertilizer are better indicative to improve teff productivity to a significant level. However there are some trials on rhizobacteria and vascular arbscular mycorrhazal using as bio fertilizer, phosphate solubilizing yeast were not studied very well. Phosphate solubilizing microorganisms can play an important role in dissolving both of fertilizer phosphorus and bound phosphorus in the soil that is environmentally friendly and sustainable.11
Several groups of microorganism including fungi, bacteria and actinomycetes are known as efficient fixed P solubilizers.12 In last few decades a large array of rhizosphere bacteria and fungi including species of Azotobacter chroococcum, Bacillus subtilis, Bacillus cereus, Bacillus megaterium, Arthro bacterilicis, Escherichia coli, Pantoea agglomerans, Pseudomonas putida, Pseudomonas aeruginosa, Entero bacteraerogenes, Microbacterium laevaniformans, and Micrococcus luteus have been identified as P-fertilizers.13
Many fungal species can solubilize rock phosphate, aluminium phosphate and tricalcium phosphate, such as Aspergillus niger, Aspergillus tubingensis, Aspergillus fumigatus, Aspergillus terreus, Aspergillus awamor, Penicillium italicum, Penicillium radicum, Penicillium rugulosum, Fusarium oxysporum, Curvularialunata, Humicola sp., Sclerotiumrolfsii, Pythium sp., Aerothecium sp., Phoma sp., Cladosporiumsp, Rhizoctonia sp., Rhizoctoniasolani, Cunninghamella spp., Rhodotorula sp., Candida sp., Schwanniomyces occidentalis, Oideodendron sp., Pseudonymnoascus sp. Candida tropicalis, Geotrichum vcandidum, Geotrichum vcapitatum, Rhodotorula minutaand Rhodotorula rubra. Saccharomyces, Hansenula, Klockera, Rhodotorula and Debaryomycesspp.,.14-20 This study was aimed to isolate, identify and evaluating of phosphate solubilizing yeast from teff rhizosphere soil collected from Gojam farm land and selecting superior solubilizing yeast that will be candidated for bio fertilizer after further evaluation for teff crop productivity.
Study area
The study was conducted in east and west Gojam in selected districts, particularly in Bichena, Bahirdar zuria, Huletejunaesae, Denbecha, Enarge enawga, Enemay, Dejenin Amhara regional state. East Gojam Zone is bordered on the south by the Oromia Region, on the west by , on the north by south Gondar, and on the east by south Wollo; the bend of the Abay River defines the Zone's northern, eastern and southern boundaries. 10˚31'44.7"N & 37˚51'10.2"E. West Gojjam (Mirab Gojjam) is one of the Zone in the Amhara Region of Ethiopia. West Gojjam is borded by North Gondar, on the north by Lake Tana, and the Abay River which separates it from the South Gondar, and on the east by east Gojjam. Coordinates: Latitude: 10.97379North, Longitude: 37.46814East.Gojam at Average altitude, 1788m.a.s.l. (Figure 1).
Sample collection
Seventy five teff farmland sites were selected based on five teff varieties, two soil types and 200 m difference within1400-1900m.a.s.l altitude in the study area. Seventy five rhizosphere soils were collected through drillings at 5, 10, and 15cm depth. Approximately 15g of soil were taken from each depth of sampling point and a total of 45g composite soil per sampling farmland were stored in sterile sample tube and icebox during November 7-17 /2016 Figure 2 and transported microbial directorate laboratory in Ethiopian biodiversity institute to Addis Ababa and kept in +4˚C until processed.
Screening and isolation of yeast from Teff rhizosphere soil
One gram of soil from each sample was serially diluted up to 10-6mL in distilled water. About 0.1mL inoculum sample was transferred to yeast extract peptone dextrose agar media (YPDA) by cotton swab and streaked using nichrom loop. Primary cultures were incubated for 26˚C in digital incubator for 48h. Isolates were subculture twice until pure colony obtained for morphological identification. A single yeast colony was streaked to Biolog universal yeast agar (BUY agar plate, (60 g/1 L) and incubated for 48h at 26˚C for yeast (YT) Microplate) inoculum preparation. The yeasts were identified according to the Biolog micro station reading procedure (Biolog, 1993).
Identification of colonial morphology
The colony morphology of the isolated yeast were examined after grown on yeast extract peptone dextrose agar media and biolog universal yeast agar media at 26˚C for 48h and its colony morphology, form, size, elevation, margin/edge, colony color were observed using hand lens and recorded.
Identification of yeast from teffrhizosphere soil using Biolog Microstation
Pure yeast isolates were transferred to biolog universal yeast agar media and incubated at 26˚C for 48h. Pure colony of yeast suspension were prepared in 9 mL sterile distilled water and adjusted to 47+2T using biolog turbidiameter.100µ-L of inoculum was dispensed using digital pipettor to each of 96wells of yeast micropate (YT) and incubated at 26˚C 24-72h. The YT micro plate is tagged with 96 carbon source. An isolate ability to metabolize each carbon source is measured in the presence or absence of purple hue in the wells. Tetrazolium violet a redox dye forms a purple color when oxidized by cellular respiration of microorganisms. The YT micro plate measures both metabolic reactions as well as turbidity growth to produce identifications. YT micro plate was read by the micro station reader at 24 h, 48 h, and 72 h at a single wavelength of 590 nm. The biolog software micro log3 ver. 4.20.05 compared the results obtained with the test strain to the database and provided identification based on distance value of match and separation score produces similarity index value and probability for species identification. Above 0.5 similarities index value result is acceptable species identification.21
Identification of phosphate solubilizing microorganisms
Yeast identified by biolog micro station was tested for their phosphate solubilizing ability. Pure yeast colony was collected using a needle nose and spot at 4 quadrants on sterile solid Pikovskaya media (2.5 g Ca3(PO4), 0.5g (NH4)2SO4, 0.2 NaCl, 0.1 g MgSO4.7H2O, 0.2g KCl, 10 g glucose, 0.5 g of yeast extract, 20 g agar, 0.0001g MnSO4, 0.0001g FeSO4, 1000 mL distilled water).22 Ca3(PO4)2 was used as a source of phosphate. Observations were made until the formation of a clear zone around the colonies of yeast that indicated the occurrence of phosphate dissolution. At 5 days intervals solubilization index (SI) was measured using following formula.23 Yeast that formed the fastest clear areas with the greatest diameter indicates the most superior phosphate solubilizing yeast.
SI = colony diameter + halozone diameter
Colony diameter
Statistical analysis
The data analysis involved various descriptive statistics such as means and percentages frequency. STATA ver.13 was used for phosphate solubilization index data analysis.
Colonial morphology
The phosphate solubilizing yeast isolates were identified based on their colony morphology depending on its pigmentation, shape, size, texture, elevation and margin. The following table summarizes the result (Table 1).
Identification of yeast species using biolog micro station
A total of 96yeast colonies were grown on yeast extract potato dextrose agar and counted. Pure colonies having similar morphology were clustered together in order to detect the percentage frequencies of the yeast. Representative yeast colony transferred into YT micro plate and read by biology microstion at 24, 48 and 72h incubation. The result revealed that nine yeast > 0.5 similarity index values were identified. These are Phichia norvegensis, Cryptococcus albidus var aerius, Candida etchellsii, Cryptococcus albidus var albidus, Rhodotrula aurantiacaA, Rhodotorula aurantiaca B, Cryptococcus luteolus, Cryptococcus albidus var diffluens, Cryptococcus terreus and three yeast isolates has no species identification result. In this study Cryptococcus were the dominant species in percentage frequency (Table 2).
Phosphate solubilization test
A total of 12yeast species were evaluated for their phosphate solubilization efficiency on Pikovskaya’s agar selective media. Among those identified by micro station, nine yeast species and 3yeast isolates with no species ID were positive for phosphate solubilization (Table 3). Their phosphate solubilization index (PSI) ranges from1.2-3.35 within 15days of incubation (Figure 4). Phichia norvegensisandCryptococcus albidus var aeriuswere superior in phosphate solubilization with great clear zone diameter and small colony diameter 3.35 and 3.2 respectively (Table 3).
P-Solubilizing Fungi |
Shape |
Elevation |
Size |
Margin |
Surface Texture |
Color |
|
1 |
Cryptococcus albidus var aerius |
Irregular |
Flat |
Large |
Lobate |
Concentric |
Yellow |
2 |
Cryptococcusterreus A |
Irregular |
Flat |
Large |
Undulate |
Smooth |
White |
3 |
Cryptococcus albidus var albidus |
Entire |
Pulvinate |
Large |
Entire |
Radiate |
White yellow |
4 |
Rhodotorula aurantiaca B |
Irregular |
Raised |
Large |
Undulate |
Concentric |
White |
5 |
Phichianorvegensis |
Circular |
Flat |
Large |
Entire |
Concentric |
White yellow |
6 |
Rhodotorula aurantiaca A |
Rhound |
Flat |
Large |
Undulate |
Radiate |
White brown |
7 |
Cryptococcus luteolus |
Circular |
Flat |
Large |
Erose |
Concentric |
Yellow white |
8 |
Candida etchellsii |
Circular |
Flat |
Medium |
Entire |
Concentric |
Yellow white |
9 |
Cryptococcus albidus vardiffluens |
Entire |
Flat |
Large |
Erose |
Concentric |
Yellow white |
Table 1 Colony morphology for phosphate solubilizing yeast
Fungus species |
Probability |
Similarity |
Distance |
Status |
Yeast Isolated from Gojam Specific Districts |
|
1 |
Rhodotorula aurantiaca A |
100 |
0.584 |
6.48 |
Identified |
Bichena, Gotera Kebele |
2 |
Candida etchellsii |
78 |
0.658 |
2.34 |
Identified |
Awabel, Enebi chifri |
3 |
Cryptococcus luteolus |
- |
0.659 |
3.19 |
Identified |
Dejen Zemetin Kebele |
4 |
Cryptococcus albidus var aerius |
100 |
0.542 |
7.22 |
Identified |
Hulet eju enese, Debre Gubae Kebele |
5 |
Cryptococcus terreus A |
99 |
0.605 |
6.03 |
Identified |
Hulet eju enese, Debre Gubae Kebele |
6 |
Cryptococcus albidus var albidus |
93 |
0.598 |
5.49 |
Identified |
Jehabitenan, Jiga Yelimdar |
7 |
Rhodotorula aurantiaca B |
86 |
0.588 |
4.86 |
Identified |
Hulet eju enese, Debre Gubae Kebele |
8 |
Phichia norvegensis |
82 |
0.52 |
5.61 |
Identified |
Hulet eju enese, Debre Gubae Kebele |
9 |
Cryptococcus albidus var diffluens |
- |
0.558 |
7.81 |
Identified |
Hulet eju enese, Debre Gubae Kebele |
10 |
Yeast isolate GTRWS18 |
- |
- |
- |
No species ID |
Hulet eju enese, Debre Gubae Kebele |
11 |
Yeast isolate GTS9B |
- |
- |
- |
No species ID |
Hulet eju enese, Debre Gubae Kebele |
12 |
Yeast isolate GTS7C |
- |
- |
- |
No species ID |
Hulet eju enese, Debre Gubae Kebele |
Table 2 Biolog micro station yeast identification result
S. No |
Fungus Species Isolated from Teff Rhizosphere Soil |
Phosphate Solubilization Index (PSI) |
||
5th days |
10th days |
15th days |
||
1 |
Phichia norvegensis |
2.51 |
3 |
3.35 |
2 |
Cryptococcus albidus var aerius |
1.32 |
2.51 |
3.2 |
3 |
Candida etchellsii |
1.76 |
2.54 |
2.90 |
4 |
Cryptococcus albidus var albidus |
2.49 |
2.57 |
2.9 |
5 |
Rhodotrula aurantiacaA |
1.16 |
1.8 |
2.4 |
6 |
Rhodotorula aurantiaca B |
1.55 |
2 |
2.24 |
7 |
Cryptococcus luteolus |
1.80 |
1.82 |
2.22 |
8 |
Cryptococcus albidus var diffluens |
1.54 |
1.67 |
1.9 |
9 |
Cryptococcus terreus A |
1.34 |
1.44 |
1.72 |
10 |
Yeast isolat GTRWS18 |
1.4 |
1.5 |
1.54 |
11 |
Yeast isolat GTS9B |
1.2 |
1.33 |
1.49 |
12 |
Yeast isolate GTS7C |
0.9 |
1.1 |
1.2 |
Table 3 Phosphate solubilization index (PSI).
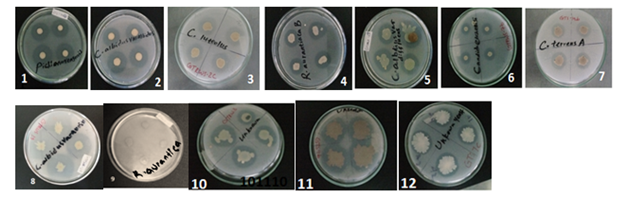
Figure 3 Phosphate solubilizing yeast on Pikovskaya’s agar media (1). Phichia norvegensis (2). Cryptococcus albidusvaralbidus (3). Cryptococcus luteollus (4). Rhodotrula aurantiaca B(5). Cryptococcus albidusvardiffluens (6). Candida etchellsii (7). Cryptococus terrusA (8). Cryptococcus albidus var aerius (9). Rhodotrula aurantiacaA (10). Yeast isolatGTRWS18 (11). Yeast isolatGTS9B (12). Yeast isolatGTS7C.
Phosphorus deficiencies are wide spread on soil throughout the world and one of the limiting factors for crop productivity. Phosphorus fertilizers represent major cost for agricultural production. Many bacteria, fungi and a few actinomycetes are potential solubilizers of bound phosphates in soil thus playing an important role making it available to plants in the soluble form.24-27 Solubilization of insoluble phosphorus by microorganisms was reported by Pikovskaya.28 During the last two decades knowledge on phosphate solubilizing microorganisms increased significantly.22 In this study a total of 96 yeast isolates were screened from teff rhizosphere soil collected from Gojam, Ethiopia and 12 yeasts were read by Microstation. Nine yeasts have got full species identification (ID) and 3 with no species ID (Table 2). All yeast species evaluated for their phosphate solubilization ability on Pikovskaya (PVK) selective media. Among all 9 yeast species and 3 isolates were positive for phosphate solubilization. Phichia norvegensis, Cryptococcus albidus var aerius, Candida etchellsii, Cryptococcus albidus var albidus, Rhodotrula aurantiacaA, Rhodotorula aurantiaca B, Cryptococcus luteolus, Cryptococcus albidus var diffluens, Cryptococcus terreus A (Table 3). Varsha et al.20 reported yeast belonging to genus Saccharomyces, Hansenula, Klockera, Rhodotorula and Debaryomyces spp. were phosphate solubilizing yeast. The soil yeasts Candida tropicalis, Geotrichum candidum, Geotrichum capitatum, Rhodotorula minuta and Rhodotorula rubra solubilized insoluble phosphate reported by Al faith.19 Delelegn Woyessa & Fassil Assefa29 reported bacteria isolated from teff rhizosphere soil from agricultural fields of Alemgena and Bushoftu Ethiopia, isolates teffrhizosphere contains a diverse flora of microorganisms. The genera were Pseudomonas, Chryseomonas, Burkholderia, Bacillus, Brevibacillus, Stenotrophomonas and Aeromonas. These 4 species Bacillus subtilis, Burkholderia cepacia, Pseudomonas fluorescens, Bacillus coagulans were superior phosphate solubilizer bacteria. However many rhizospheric bacteria and fungi isolated from different crop rhizosphere soil, there is little information regarding teff rhizophere yeast and potential phosphate solubilizer yeast. This study will confirm that there are a diverse teff rhizosphere yeast and superior phosphate solubilizer isolated from Gojam teff farm land (Table 3). The yeast species Rhodotrula aurantiaca A are phosphate solubilizer fungi species discovered in this study are also similar with the work of Yasser et al.30 and Isbelia et al.14 In this study phosphate solubilization index (PSI) were measured within 5 days intervals for 15 days of incubation and they measured 1.2 -3.35PSI clear zone diameter over colony diameter ratio (Table 3). Narsian et al.31 reported yeast belonging to genus Saccharomyces Hansenula, Klockera, Rhodotorula and Debaryomyces exhibited highest SI (1.33-1.50). The study by Yasser et al.30 phosphate solubilization index recorded 1.05-1.45. A .japonicas (SI=1.45), A. niger (SI=1.12), Penicillium expansum (SI=1.20), Penicillium funiculosum (SI=1.40), Penicillium variable (SI=1.13), Penicillium purpuragenum (SI=1.30). In this study the largest solubilization index recorded by Phichia norvegensis(SI. 3.35), Cryptococcus albidus var aerius(SI.3.2), Candida etchellsii (SI.2.9).The smallest solubilization index recorded by Cryptococcus terrus A (PSI, 1.72) (Figure 3 & Table 3). According to De Freitas, good phosphate solubilizers produce halos around their colonies with diameters higher than 1.5 cm. Most efficient phosphate solubilizer on Pikovskaya’s agar plates with PSI = 3.29. Whereas among fungi P. canescenssho wed highest solubilizing index Nahas.32 Phosphate solubilization index (PSI) values up to 2.4 have been recorded for Aspergillus niger, with values of 3.1 for Penicillium italicum and 3.0 for Paecilomyces lilacinus .33,34 Fungal strains isolated from sugarcane and sugar beet rhizosphere showed SI in range of 1.13 to 1.59 Mahamuniet et al.35 Alam et al.36 reported PSI of the fungal strains isolated from maize rhizosphere that ranged from 1.53 to 1.80. In this study new phosphate solubilizer yeast Phichia norvegensis, Cryptococcus albidus var aeriusidentified from teffrizhosphere soil with superior solubilization index (PSI) 3.35 and 3.2 respectively in 15 days incubation.37,38 Therefore, these strains can be candidated and exploited as bio fertilizers through further evaluation and optimization test to increase agricultural productivity of teff crop.
Ninety six yeasts were screened from teffrhizosphere soil and morphologically similar isolates were clustered and representative yeast isolates were identified by biolog microstation identification system where equivalent to molecular techniques and the dominant species were Cryptococcus species. Nine yeast species and 3 yeast isolates Phichia norvegensis, Cryptococcus albidus var aerius,Candida etchellsii, Cryptococcus albidus var albidus, Rhodotrula aurantiacaA, Rhodotorula aurantiaca B, Cryptococcus luteolus, Cryptococcus albidus var diffluens, Cryptococcus terreus A, Yeast isolate GTRWS18, GTS9B, GTS7C were positive for phosphate solubilization ability. Phichia norvegensis and Cryptococcus albidus var aerius were superior among the isolated fungi in solubilizing index 3.35 and 3.2respectively and good candidate for biofertilizer after further evaluation on in vitrotest, green house and field trials. The rise in the cost of chemical fertilizer, the lack of fertilizer industries in developing countries and the growing environmental issue and biodiversity loss using chemical fertilizer timely important concern using alternative ecofriendly bio fertilizer to increase yield and productivity of teff crop.
Utilization efficiency of crops for phosphate chemical fertilizer is around 30%, the remaining 70%exist in compound and bound form, Such economic considerations and phosphate existence in compound form necessitate for an alternative less expensive and environmentally friendly bio fertilizer improving yield and quality of teff grain
The benefficial effects of plant growth promoting microorganisms(PGPM) have not been exloited well. In the past some microbial inoculants prepared from Rhizobium for leguminous crops, Azotobacter and Azospirillium for cereal crops and Frankia for tree crops have been used as nitrogen providers in many developed and developing countries. However enormous interest increase in research in recent years in PGPM such as nitrogen fixer, phosphate solubilizer, pathogen suppressor.There is no well-organized microbial inoculant industry for bio fertilizer production especially for phosphate solubilizer and there is no link with researcher working on microbial bio fertilizer in Ethiopia, there for agricultural research institute, microbiologist, soil scientist agronomist, and stockholders in general must work together in depth on structural and functional diversity of PGPM and selecting superior biofertilizer, biopesticide, biostimulant to increase crop yield and prductivity. Further research should be continued with selecting efficient phosphate solubilizer microorganism (PSM) isolates. These may be used for inoculum production and their inoculation effect on the plant growth must be studied in vitro, green house and field trials.
It gives me a great pleasure to acknowledge Dr. Genene Tefera for his unreserved guidance and encouragement and support in providing and facilitating the necessary equipment and extremely greatful to acknowledge Gojam zonal and district agriculture office and leader of all area in the research area who helped me guiding the study site and finally goes to Ethiopian biodiversity institute, microbial directorate for every budget grant to carry out this study and its research team for their un reserved support at laboratory and field work especially Misganaw Wasie, Endeshaw Abatneh, Endegena Aynalem, as part of research group in tireless effort in teffrhizospher soil sample collection. Lastly I acknowledged Woyenshet Lule for her kindly support especially laboratory chemicals facilitation.
The author declares no conflict of interest.

©2017 Gizaw, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.