Journal of
eISSN: 2469 - 2786


Research Article Volume 6 Issue 6
Microbiology Laboratory, Biotechnology Research Department, Myanma
Correspondence: Zaw Ko Latt, Microbiology Laboratory, Biotechnology Research Department, Myanmar, Tel +95 9 973084176
Received: December 05, 2018 | Published: December 28, 2018
Citation: Thant S, Aung NN, et al. Phosphate solubilization of Bacillus megaterium isolated from non-saline soils under salt stressed conditions. J Bacteriol Mycol Open Access. 2018;6(6):335-341. DOI: 10.15406/jbmoa.2018.06.00230
Phosphate solubilizing Bacillus megaterium was isolated from non rhizospheric soils. Five isolates were identified by 16s rDNA sequencing. According to sequencing and phylogenetic tree analysis, these five isolates were in high similarity with B. megaterium. Abiotic stresses such as temperature, pH and salt tolerance of five strains were examined for the application for abiotic stressed agricultural soils. All five strains could grow well above 40ºC. For pH, five strains demonstrated their growth on minimal media at pH 9 but the growth rates were decreased. Among abiotic stress factors, to obtain salt tolerance phosphate solubilizing B. megaterium was the main objective of this research work. So, salt tolerance of isolates were screened preliminary, all five strains had the ability to grow on minimal media containing 6% NaCl concentration but the growth rates were not in high rates. Preliminary screening results enhanced to continue for quantative determination of phosphate solubilization under salt stressed conditions. In detection of phosphate solubilization by the method of Olsen P, all five strains could solubilize insoluble phosphate, Tricalcium phosphate, under NaCl stressed conditions. Among 4%, 5% and 6% NaCl concentrations, soluble phosphate concentrations released by five strains were decreased with increasing NaCl concentrations, but it was not obviously different.
Keywords: phosphate solubilization, Bacillus megaterium, abiotic stress, yemperature, pH, salinity
Soil is a major source of nutrients needed for the growth of plants. Nitrogen (N), phosphorus (P) and potassium (K) are the three main nutrients required for the plants. Together they make up the trio known as NPK. Plants use these three nutrients in large amounts for their growth and survival and so these major nutrients usually are lacking from the soil first.
Nitrogen, a key element in plant growth, is found in all plant cells, in plant proteins and hormones, and in chlorophyll. Atmospheric nitrogen is a source of soil nitrogen.
Soil nitrogen is largely obtained from the atmosphere by the help of microbes. With the help of nitrogen fixing microbes, plants obtain ammonia as nitrogen source from soils.
Phosphorus is found in soils both in an organic form and an un-organic (mineral) form and its solubility in soil is low. There is equilibrium between solid phase phosphorus in soil and the phosphorus in the soil solution.
Plants can only take up phosphorus dissolved in the soil solution, and since most of the soil phosphorus exists in stable chemical compounds, only a small amount of phosphorus is available to the plant at any given time. When plant roots remove phosphorus from the soil solution, some of the phosphorus adsorbed to the solid phase is released into the soil solution in order to maintain equilibrium.1
Plant growth is actually controlled by various factors; especially root temperature and nutrient supply are the main factors. In particular, nutrient uptake is affected by soil temperature2 that may influence the physico-chemical and biological processes which affect nutrient availability in soils, and in turn affect plant nutrient uptake.3
Microorganisms play an important role in the growth and development of plants whether the environmental conditions are severe or not. Microorganisms from any sources are often exposed to biotic stresses, viz., pathogens, foreign metabolites, as well as environmental stresses, viz., temperature, pH, moisture content, nutrient availability, etc.4 Therefore, they have a wide range of mechanisms for adaptation to sense and respond the stresses. Moreover, they can modify their signal transduction networks to cope with stress conditions.
Therefore, most farmers have turned their attention to use of plant growth promoting bacteria for biotic and abiotic stresses that will potentially occurred due to climate change. Nevertheless, the performance of PGPR as biofertilizers is severely influenced by both biotic and abiotic environmental conditions of the targeted regions. This has lead researchers to seek out isolation of PGPR from native soil and to explore methods for screening PGPR strains and subsequent evaluations for specific criteria particularly with respect to applications in various environmental conditions.
Several strains of bacterial and fungal species have been described and investigated in detail for their phosphate-solubilizing capabilities.5,6 Typically such microorganisms have been isolated using cultural procedures with species of Pseudomonas, Bacillus bacteria,7 Aspergillus and Penicillium fungi being predominant.8 Bacillus and other members of this genus were dominant salt-tolerant plant growth-promoting rhizobacteria, which could serve as a suitable bio-inoculant for crops grown under saline soils.9‒12
In this research work, B. megaterium was a main candidate strain as phosphate solubilizers and their abilities were evaluated for abiotic stress tolerance. Salt tolerance activity was mainly emphasized and so, phosphate solubilizaing ability under salt stress conditons was determined.
B. megaterium is a gram positive and rod shaped bacterium, and produces endospores, plant growth promoting substances and inhibitory molecules.13 Phosphate solubilization ability of B. megaterium was reported by several researcher,14 but optimal conditions for efficient phosphate solubilization of B. megaterium is still lacking.
B. megaterium grows at temperatures from 3ºC to 45ºC, with the optimum around 30ºC. Some isolates from an Antarctic geothermal lake were found to grow at temperatures up to 63ºC.15
B. megaterium is known to produce poly-γ-glutamic acid. The accumulation of the polymer is greatly increased in a saline (2–10% NaCl) environment, in which the polymer comprises largely of L-glutamate (L-isomer content up to 95%).16 At least one strain of B. megaterium can be considered a halophile, as growth on up to 15% NaCl has been observed.17
The objectives of this research work are to obtain salt tolerant B. megaterium from various soils of agricultural land in our country and to evaluate the potential of isolated B. megaterium as biofertilizer for abiotic stressed agricultural soils in our country.
Soil sample collection
Soil samples from agricultural soils that were planted by different crops such as rice, sesame, pepper and corn, were collected from 15 cm depth of upper soil surface around Kyaukse District, Mandalay Region, Myanmar.
Isolation of B. megaterium
One gram of soil samples was transferred into 50mL sterile test tube containing 10mL of sterile 0.9% NaCl solution. The soil suspensions were stand for about one hour and serially diluted. 100µL of aliquots was spread on minimal media. Composition of minimal media was as follow; g/L: K2HPO4 2.5g, KH2PO4 2.5g, (NH4)2 HPO4 1g, MgSO4 0.2, FeSO4 0.01g, MnSO4 0.007g, Sucrose 10g) and incubated at 30ºC for 48hrs.
Purification of B. megaterium
The bacterial colonies appeared on minimal media were transferred onto nutrient media for differentiation of different color of colonies. Single colonies on nutrient media were transferred onto minimal media again and purified by subculturing.
Characterization of B. megaterium
Isolated strains were characterized by colonial and microscopic morphology, some biochemical characteristics. Single colony of isolated strains were cultured on minimal media and incubated at 30ºC for 48hrs. Colonial morphology was observed. Microscopic morphology was examined by gram staining method.
Identification by 16s rDNA sequencing and phylogenetic tree analysis
Isolated bacteria were identified by 16S rDNA sequencing. DNA extraction was performed using the PrepMan reagent (Thermo fisher Scientific, California, US) and primers 10F (5'-AGTTTGATCCTGGCTC-3') and 800R (5'-CTACCAGGGTATCTAAT-3'). 16S rDNA was amplified by PCR 30 cycles using a universal primer. Each cycle consisted of denaturation for 1 min at 94ºC, annealing for 30 s at 56ºC, and extension for 45 sec at 72ºC and final extension for 45 sec at 72ºC. Sequencing reactions were performed using the Big Dye Terminator Cycle Sequencing Kit (v.3.1) (Thermofisher Scientific, California, US) and analyzed in Genetic Analyzer 3130 sequencer (Applied Biosystems, US). Nucleotide sequences were analyzed using BLAST on the NCBI BLASTN and Greengenes.lbl.gov.
Nucleotide sequences of five strains of B. megaterium were analyzed by BLAST, presenting the highest sequence homology proportion, query coverage, and the lowest E values. The closely related sequences were aligned by ClustalX using MEGA version 6.0 software, and the neighbor-joining tree was generated using same software. Bootstrap replication (500 replications) was used for a statistical support for the nodes in phylogenetic tree. The sequence of other genera was also used to determine the actual relationship among participating candidates in the group.
NaCl tolerance of B. megaterium
NaCl tolerance of B. megaterium was screened on minimal media containing different concentrations of NaCl (3%, 4%, 5%, 6%, 7%). Incubation temperature and period were the same for NaCl tolerance screening.
Temperature and pH tolerance
Temperature tolerance of five isolates was examined by culturing on minimal media and incubation at different temperature such as 40ºC, 41ºC, 42ºC, 43ºC, 44ºC, 45ºC. For pH tolerance, single colony of bacteria was cultured on minimal media that was adjusted by different pH and incubated at 30ºC.
Phosphate solubilization of B. megaterium under salt stressed conditions
Phosphate solubilization of isolated B. megaterium was studied by method of Olsen et al., 1954. Single colony of B. megaterium was inoculated into 10mL of Pikovskaia’s18 broth containing 4%, 5% and 6% NaCl concentrations and incubated in water bath shaker at 30ºC. Tricalcium phosphate was used as substrate. Solubilization of tricalcium phosphate was measured at 3, 5 and 7 days. The bacterial broth was centrifuged at 5000rpm for 5mins and 5mL of supernatant was transferred into 50mL centrifuge tueb. 2.5mL of freshly prepared 1M HCL solution was added and then a little water was added. 2.5mL of molybdium solution was added and mixed well. Then water was added to make the final volume of 25mL and the mixture was stand for 30mins. Absorbance was measured at 882nm after half an hour. A standard curve was prepared using 0, 1, 2, 3, 4 and 5ml of 5ppm standard P solution.
Isolation, identification by 16s rDNA sequencing and phylogenetic tree analysis
In soil, the constituent of phosphate solubilizing bacteria was 1-50% and 0.1-0.5% for fungi of the total respective population. They are generally isolated from rhizosphere and non rhizosphere soils, rhizoplane, phyllosphere, and rock phosphate deposit area soil and even from stressed soils using serial plate dilution method or by enrichment culture technique.19 In this study, phosphate solubilizing bacteria were isolated from non rhizospheric soils.
According to 16s rDNA sequencing analysis, all five isolates were in high similarity with B. megaterium after comparing the sequence homology using BLAST. It was also confirmed by examining the colonial and microscopic morphology of five isolates and the findings showed that the morphology of five isolates was confirmed as B. megaterium.
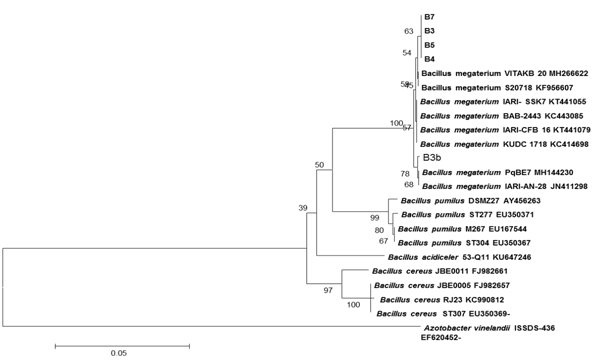
The phylogenetic tree analysis also proved that isolated five strains of phosphate solubilizing bacteria were identified as B. megaterium on the basis of sequence similarity. Constructed phylogenetic tree of five strains of B. megaterium was shown in Figure 1.
Antibiotic sensitivity of B. megaterium
Antibiotic sensitivity of five strains of B. megaterium was detected using four antibiotics (ampicillin, chloramphenicol, kanamycin and tetracycline). Their sensitivity patterns were described in Table 1.
No. |
Strains |
Antibiotics |
|||||||
Ampicillin |
Chloramphenicol |
Kanamycin |
Chloramphenicol |
||||||
1 |
B3 |
26mm |
S |
29mm |
S |
36mm |
S |
37mm |
S |
2 |
B4 |
29mm |
S |
34mm |
S |
35mm |
S |
37mm |
S |
3 |
B5 |
29mm |
S |
32mm |
S |
34mm |
S |
37mm |
S |
4 |
B7 |
28mm |
S |
34mm |
S |
36mm |
S |
36mm |
S |
5 |
B3b |
26mm |
S |
31mm |
S |
34mm |
S |
39mm |
S |
Table 1 Antibiotic sensitivity patters of five strains of B. megaterium
S- Sensitive
Five strains of B. megaterium were sensitive to all used antibiotics. Nowadays, antibiotic resistant strains may have evolved naturally, and so, it has to be worried for the safety to use in agriculture. So, all strains that will potentially be used for agriculture need to be checked for their antibiotic sensitivity patterns for some commonly used antibiotics.
Temperature and pH tolerance
The threat of climate change is increasing and due to the increasing threat of climate change, agricultural production worldwide has been threatened by substantial impact. Actually, heat waves cause significantly yield losses with great risks for future global food security.20
Temperature and pH tolerance of five isolates of B. megaterium were screened for other abiotic stresses. On minimal media, all five isolates were cultured and incubated at different temperature. Above 42ºC, the growth rates of some isolates were decreased and all isolates could not grow at 45ºC except B3b.
Recent reports suggest that the tolerance of plants to temperature stress can be enhanced by PGPR.21‒ 23 Among the abiotic stresses, temperature is one the factors that greatly influence the phosphate solubilizing potentials of microbes.24 There has been a conflicting report on the influence of temperature on phosphorus solubilization by microbes.
B. megaterium was isolated by incubating the culture media at 30ºC. When incubation temperature was increased from 30ºC to 44ºC, it was found that all five isolates could grow well until 44ºC. So, environmental temperature around 44ºC cannot affect the growth of isolates and it can be said that isolated phosphate solubilizing bacteria can be used as temperature tolerance strains for agricultural sector of our country.
In addition, isolated five strains of B. megaterium could grow on minimal media that was adjusted to pH 9. But the growths of these strains were less. It was described in Table 2.
No. |
Strains |
Different Temperature (from 40ºC to 45ºC) |
Different pH |
|||
40ºC |
44ºC |
pH 5 |
pH 7 |
pH 9 |
||
1 |
B3 |
+++ |
+++ |
- |
++ |
+ |
2 |
B4 |
+++ |
++ |
+ |
++ |
+ |
3 |
B5 |
+++ |
+++ |
+ |
+++ |
++ |
4 |
B7 |
+++ |
+++ |
+ |
+++ |
+ |
5 |
B3b |
+++ |
+ |
+ |
+++ |
+ |
Table 2 Temperature and pH tolerance of five strains of B. megaterium
Screening on Salt Tolerance
Isolated B. megaterium can grow well in variation of pH value of the media. It was seemed that these five isolates seem to adapt to temperature and pH variation for their growth.
Among abiotic stress factors, soil pH is one of the factors influencing microbial phosphate solubilization.25 Soil pH values between 6 and 7.5 are best for P-availability. Isolated B. megaterium tolerated pH in the value of 9 and it can be thought that these five bacteria can grow well and function for phosphate solubilization in high pH soil types.
It has been estimated that worldwide 20% of total cultivated and 33% of irrigated agricultural lands are afflicted by high salinity.26 The impacts of salinity include less agricultural productivity, less economic returns, and soil erosions.27, 28 Bacteria-induced salt tolerance in plants has been observed for several PGPR strains.29‒32
Salt tolerance of isolated B. megaterium was screened on minimal media containing different concentrations of NaCl. At 3% NaCl concentration, all isolated B. megaterium grew well, and their growth was not different when detected visually. But from 4% to 6% NaCl concentrations, the growth rates of five strains of B. megaterium were not the same. Some strains grew better than others. At 7 % NaCl concentration, all strains did not grow except B3b. It was described in Table 3.
No. |
Strain Code |
Salt concentration |
||||
3% |
4% |
5% |
6% |
7% |
||
1 |
B3 |
+++ |
++ |
++ |
+ |
- |
2 |
B4 |
+++ |
++ |
++ |
+ |
- |
3 |
B5 |
+++ |
++ |
++ |
+ |
- |
4 |
B7 |
+++ |
++ |
++ |
+ |
- |
5 |
B3b |
+++ |
+++ |
+++ |
+ |
+ |
Table 3 Growth rates of isolated B. megaterium on minimal media containing different concentrations of NaCl.
Quantitative Determination of Phosphate Solubilization under Salt Stressed Conditions
All five isolates were tolerant to and grew well until 6% NaCl concentration but their growth rates were different. Although these all five strains were isolated from Kyaukse District, Myanmar, and identified as B. megaterium, their mechanism for the growth under abiotic stressed conditions may be different. When the NaCl tolerance of these strains were compared with other phosphate solubilizing bacteria from previous reported research works, it was noticed that their tolerance were less.
In this research work, phosphate solubilizing bacteria were isolated from agricultural soils that were not stressed by high salinity. Other researchers studied the influence of salts on the phosphate solubilizing ability of bacteria, but they isolated phosphate solubilizing bacteria from salt affected soils. Srinivasan et al.33 studied the effects of salt on survival and phosphate solubilization potential of microorganisms isolated from salt affected soils. Surange et al.34 had studied the NaCl tolerance and phosphate solubilization of the Rhizobium strains isolated from alkaline soils.
Phosphate solubilizing abilities of isolated B. megaterium were determined under salt stressed conditions. With increasing NaCl concentrations, the phosphate solubilizing activities were decreased but it was not obviously different among different NaCl concentrations for phosphate solubilization. Most strains increased their solubilizing activities after 3 and 5 days incubation. Phosphate concentrations solubilized from tricalcium phosphate by all strains were shown in Figure 2.
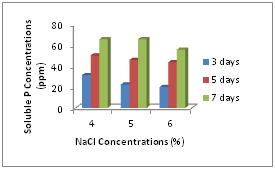


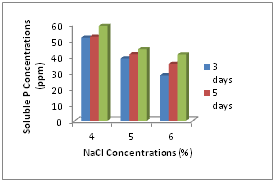
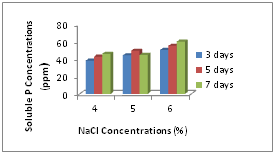
In quantitative determination for phosphate solubilization, we found the similar results like other research. Their solubilization abilities for insoluble phosphate were deceased with increasing NaCl concentration. Increasing NaCl concentrations effect on the growth rates of bacteria, and so, decreasing the growth rates of bacteria caused decreasing of phosphate solubilization. But it was obviously found that their solubilization ability was increased after taking some time for their growth. So, it can be said that phosphate solubilizing activity of bacteria can be increased under abiotic stressed conditions after adapting to conditions.
Effective existence of bacteria in saline environment due to excessive accumulation of secondary metabolites may result in better root colonization and plant growth.35,36 According to the findings of Habib et al.37 two PGPR B. megaterium and Enterobacter sp. induce salt tolerance and consequent improvement in the growth of okra plants under salt stress. Ramadoss et al.38 reported that halotolerant bacteria Hallobacillus sp. SL3 and Bacillus halodenitrificans PU62 isolated from saline environments have potential to enhance plant growth under saline stress.
Phosphate solubilizing microorganisms that are isolated from soils that are influenced by environmental extremes such as saline-alkaline soils, soil with a high level of nutrient deficiency, or soil from extreme temperature environments have the potential to solubilize more phosphate than phosphate solubilizing microorganisms from soils from more moderate conditions.39
Surange et al.34 had reported that the Rhizobium strains isolated from alkaline soils tolerated salt concentrations up to 860mM (5.0%), but the growth of Rhizobium strains was inhibited by a concentration of 1290 M (7.5%). Some researchers reported that phosphate solubilizing ability of some bacteria decreased with an increase in NaCl concentrations. In contrast, some found that the phosphate solubilization of some bacteria increased with an increase in NaCl concentrations but there is a limit in NaCl concentration that did not affect the ability of bacteria. Kumar et al.40 also found increased phosphate solubilization with an increase in NaCl concentration.
Srinivasan et al.33 reported that the amount of Pi released from tricalcium phosphate by the phosphate solubilizing bacterial isolates was increased until salt concentrations up to 800mM NaCl. But he also reported that after this concentration, the phosphate solubilizing ability of bacteria was decreased.
In our study, the growth and phosphate solubilizing abilities of five strains of B. megaterium was influenced by NaCl. With increasing NaCl concentrations, the growth of bacteria was less and less. These five strains tolerated to NaCl concentrations up to 6%. But with longer incubation period, the amount of soluble phosphate from tricalcium phosphate by five strains of B. megaterium was increased. It can be thought that bacteria need to adapt to higher NaCl concentrations that their normal needs to grow and function for phosphate solubilization. After adapting the NaCl concentraions containing in media, they start to grow well and function.
Due to increasing Temperature, pH changes and increasing salinity of soil our country, abiotic stress tolerance plant growth promoting bacteria are becoming the first priority to isolate and assess their plant growth promoting activity under abiotic stressed conditions. Phosphate solubilizing B. megaterium was isolated and examined their abiotic stress tolerance and their phosphate solubilizing ability under NaCl stressed conditons. Five strains of B. megaterium were isolated and these all five strains have demonstrated to grow at different temperature, pH and NaCl concentrations that will be possibility occurred for agricultural sectors in our country. But their tolerance for above three factors was not extremely high. According to in vitro tests, these five strains of B. megaterium could be applied as phosphate solubilizers for abiotic stressed soils.
None.
There is no conflicts of interest among the authors.

©2018 Thant, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.