Journal of
eISSN: 2469 - 2786


Research Article Volume 4 Issue 4
Industrial Waste Water Research Laboratory, India
Correspondence: Maulin P Shah, Industrial Waste Water Research Laboratory, Applied & Environmental Microbiology Lab, Enviro Technology Limited (CETP), Plot No: 2413/2414, GIDC, Ankleshwar- 393 002, Gujarat, India
Received: December 14, 2016 | Published: April 17, 2017
Citation: Shah MP. Microbial decolorization of mixture of dyes by an application of laccase through bacillus subtillis ETL-1979 isolated from soil of industrial effluent treatment plant. J Bacteriol Mycol Open Access. 2017;4(4):115-119. DOI: 10.15406/jbmoa.2017.04.00099
The strain bacillus subtillis. ETL-1979 exhibiting laccase activity was screened from industrial effluent treatment plant. The M9 medium containing Cu2+ was used for enriching and isolating bacterial strains capable of oxidizing syringaldazine. One isolated strain was identified as Bacillus subtilis ETL-1979 based on the results of physiological and biochemical tests and 16S rDNA sequence analysis. The strain ETL-1979 could grow at temperatures ranging from 20 to 55°C and showed optimum growth temperature and pH at 25°C and 7.0, respectively. The sporulation rate of the strain clearly correlated well with the laccase activity. The temperature half-life of the spore laccase was 2.5h at 80°C and the pH half life time was 15d at pH 9.0. Its spore laccase could decolorize 50-90% of Remazol brilliant blue R (RBBR), alizarin red, congo red, methyl orange and methyl violet, which suggests the potential application of spore laccase in dyestuff treatment.
Keywords: bacterial laccase, bacillus subtilis, spore, decolorization
Laccases (benzenediol: oxygen oxidoreductases, EC1. 10.3.2) are multi-copper proteins that can oxidize a wide range of inorganic and aromatic compounds, particularly phenols, while reducing molecular oxygen to water.1 Laccases catalyze the removal of a hydrogen atom from phenolic substrates and aromatic amines by one-electron abstraction. The free radicals formed during the reaction are also capable of undergoing further depolymerization, repolymerization, demethylation or quinone formation.2-4 The low substrate specificity of laccases and their ability to oxidize many pollutants suggest their industrial and biotech-nological applications.5,6 Laccases are widely distributed among fungi and plants.7 However, it has been found that laccases are also widespread among bacteria.1 To date, laccases have mostly been isolated and characterized from plants and fungi but only fungal laccases are used currently in biotechnological applications. In contrast, only a few bacterial laccases have been characterized. Bacterial laccases could oxi-dize syringaldazine and 2,6-dimethoxyphenol, which are typical substrates for laccases, and also possess the canonical four copper-binding domains. Nervertheless, the overall sequences of bacterial laccases show little similarity with fungal laccases. Therefore, they are often known as “multicopper oxidase” or “(poly) phenol oxi-dase”, and their activity is usually defined as “laccase-like”.8 The first report on bacterial laccase was from the non-motile strain of Azospirillum lipoferum isolated from rice rhizosphere.9 This enzyme was identified as a laccase by using a combination of substrates and inhibitors.1,10 Laccases activities were also found in Bacillus sphaericus,11 Escherichia coli,12 Bacillus halodurans13 and Streptomyces. psammoticus .14 CotA, which is the endospore coat component of Bacillus subtilis, is the most-studied bacterial laccase.15 Since spores allow microorganisms to survive under drastic conditions, spore coat enzymes might also withstand high temperatures or extreme pH values. Since most fungal laccases are unstable at pH values higher than 7.0, their detoxification efficiencies for pollutants often decrease under alkaline conditions. This limits the industrial potential of fungal laccase as many processes are performed in alkaline conditions. Alterna-tively, spore laccases which are active in the alkaline pH range could be used for bioremediation or application in membrane reactors.4 Compared with fungal laccases, bacterial laccases have the advantages of being less sensitive towards halides and alkaline conditions as well as a fast growth rate.16 Despite the importance of bacterial laccase in pollutant degradation, only a few novel bacterial strains displaying “laccase-like” activity have been discovered. The lack of a commercially available robust and inexpen-sive laccase is a major barrier to the widespread application of laccases in various industrial sectors.17 Since bacterial genetic tools and biotechno-logical processes are well established, developing bacterial laccases would be significantly important.18 The present study was carried out to isolate and characterize the bacteria strain Bacillus sp. ETL-1979 exhibiting laccase activity from soil of industrial effluent treatment plant. The spore laccase of this strain was characterized and used to decolorize several synthetic dyes.
Sample collection
The soil samples used in the present study were collected from textile effluent samples from textile industry, Ankleshwar, Gujarat, India. Collected soil samples were stored aerobically at 4˚C.
Isolation of microorganisms
For isolation and enrichment of laccase-producing bacterial strains, the 250-ml flasks containing 100ml M9 culture medium supplemented with 0.2mmol/l Cu2+ were inoculated with 10g of soil particles and incubated at 37˚C on a rotary shaker (130 rpm) for 2 days. Then 5 ml cultures were transferred to 100ml Luria-Bertani (LB) culture medium containing 0.2mmol/l Cu2+ and incubated at 37˚C at 130 rpm for 7 days. Stable enrichment cultures were obtained after subculture. To isolate pure cultures, the enriched cultivated products were appropriately diluted with sterile saline solution (0.9% NaCl) before spreading onto LB/Cu2+ plates. The plates were incubated at 37˚C for 3 days. Individual bacterial colonies from the plates were drop-ped with 0.1% (m/v) syringaldazine for checking laccase activity. The colonies displaying pink were streaked on new LB/Cu2+ plates for purification. Re-inoculation was performed after identification with syringaldazine as described above. The isolation process was repeated several times until the isolates were found to be pure.
Characterization
Gram staining was performed according to standard protocol. Gram’s characteristics and cell morphology of the isolated strain were determined by microscopy. For carbon sources utilization, the pure cultures were inoculated respectively into peptone-water culture medium containing 1% substrates, and incubated at 37˚C for 24h. The results were determined by the variation of both the turbidity and the color of the culture medium. Control tubes con-tained uninoculated medium and were incubated at the same conditions. Some biochemical metabolic ability was determined by inocula-ting isolates onto selective media such as casein, urea, balsam and starch agar, to identify protease, urase, lipase and amylase pro-ducers, respectively. All assays were carried out in triplicate.
Molecular characterization
Bacterial cells were collected by centrifugation at 10,000 rpm for 2 min and incubated with 100μg/ml lysozyme at 37˚C for 1 h followed by the treatment of lysis solution (1% SDS, 1mmol/L EDTA, 20mmol/l CH3COONa and 40mmol/l Tris-HCl, pH 8.0). After adding 5mmol/l NaCl into the lysis solution, the mixture was extracted with phenol/chloroform/isoamyl alcohol (25:24:1). The supernatant was collected and then precipitated by absolute ethanol. The obtained genomic DNA was dissolved in sterile deionized water and stored at -20˚C for further use. For polymerase chain reaction (PCR), primers specific for eubacterial 16S rDNA 27F: 5’-GAGTTTGATCMTGGCTCAG-3’ (M=A+C), 1492R: 5’-TACGGYTACCTTGTTACGACTT-3’ (Y=C+T) were used.19 The PCR was run in a Gene Amp PCR system 9700 (Applied Biosystems, Singapore). The amplification reaction consisted of an initial denaturation at 93˚C for 5 min, followed by 30 cycles of 94˚C for 18 s, 56˚C for 15 s and 72˚C for 78 s, and a final extension step at 72˚C for 7 min. The PCR products were analysed by electrophoresis in 1.0% (w/v) agarose gel and photographed using Bio Imaging System (Gene Genius, USA). Amplification products were cloned using a commercially avail-able pMD18-T vector cloning kit and transformed into competent E. coliJM109. Positive clones were identified by PCR amplification with above primer pairs.
Nucleotide sequencing, alignment and phylogeny
16S rDNA sequencing of the isolated strain was done by Bangalore Genei Company, India. Related sequences were obtai-ned from the GenBank database using the Basic Local Alignment Search Tool (BLAST) search program. Multiple sequences alignment was performed using Clustal X 1.81. Phylipwx package was used to calculate similarity values and construct a phylogenetic tree.
Optimization of growth conditions
The optimal growth conditions with regard to pH and temperature were determined. The strain was inoculated in LB medium with varying pH values (4 - 11) and incubated at 15 - 55˚C. The optical density of the growing cultures was observed at 600 nm using UV-1800 spectrophotometer (Shimzadzu, Japan) to determine the optimum growth conditions. All assays were carried out in triplicate.
Effect of metals and saline solution on bacterial growth
To study the effect of metals on growth, 200μg/ml Zn2+, Fe3+, Ca2+, Mn2+, Mg2+ or Cu2+ was supplemented in LB culture medium, respectively. Cultures were grown in 25ml medium in 100 ml conical flasks at 37˚C for 24 h. Culture grown in absence of metal was used as a control. Growth was determined by measuring the absorbance at 600 nm against blank. The strain was inoculated in LB medium supplemented with 1, 2, 4, 6, 8, 10 or 12% NaCl. The turbidity of the growing cultures was observed at 600 nm using UV-1800 spectrophotometer (Shimzadzu, Japan) to determine the growth status. All assays were carried out in triplicate.
Sporulation rate and laccase activity relationship
B. subtilisETL-1979 was inoculated on LB plates containing 0.2mmol/l Cu2+ and incubated at 30˚C. The quantities of the spore were calculated every day and the laccase activity was determined at the same time. The sporulation rate was determined by the percentage of the quantities of spore opposite to all cells. The spores were removed from the agar with 1mol/l KCl, washed with 0.5mol/l NaCl, and re-suspended in 0.1 mol/l citrate-phosphate buffer (pH 6.8). The spore suspension was prepared for laccase activity determination. All assays were carried out in triplicate for each sample.
Spore laccase activity assay
Spore laccase activity was determined at 40˚C using syringaldazine (dissolved in absolute ethanol, Sigma) as the substrate. The oxidation of syringaldazine was detected by measuring the absorbance increase at 525 nm (ε525 = 65 mmol-1 L cm-1) after 3 min using a spectrophotometer (UV-1800 spectrophotometer, Shimzadzu, Japan). The reaction mixture (3ml) contained 100μl of spore suspension (10mg wet spores), 2.4 ml of citrate–phosphate buffer (0.1mol/L, pH 6.8), and 0.5 ml of 0.5mmol/L syringaldazine. One unit of enzyme activity was defined as the amount of enzyme required to oxidize 1μmol of substrate per minute. All assays were carried out in triplicate for each sample. Standard deviation did not exceed 5% of the average values.
Effect of pH and temperature
Determination of the optimum pH was conducted in 0.1 mol/l citrate–phosphate buffer in the range of pH 4.0 – 8.0 using syringaldazine as the substrate. The optimum temperatures of the spore laccase were determined over the range of 0 – 100˚C with syringaldazine as the substrate at their optimum pH values. All assays were carried out in triplicate. Thermal stability of the spore laccase was determined by preincubation of 0.1mol/L citrate-phosphate buffer (optimum pH) of spores at 60 and 80˚C and the remaining activity was measured with the assay described above. The pH-stability was examined similarly at 30˚C in different buffers ranging from pH 4.0 to 9.0. All assays were carried out in triplicate.
Determination of dye decolorization efficiency
The general dyes, remazol brilliant blue R (RBBR), alizarin red, congo red, methyl orange and methyl violet, were prepared individually with the concentration of 25mg/l in sterilized distilled water. The prepared dye solution was supplemented with 100g/l spores and incubated at 37˚C under mild shaking conditions for 5 days. Dye samples without spores were given the same treatment as the control. The absorption spectrum of each dye between 200 and 800 nm was measured with a UV-1800 spectrophotometer (Shimadzu, Japan). Dye decolorization was assessed by the decrease in absorbance under the maximum wavelength of the dye. All assays were carried out in triplicate.
Isolation of bacterial strain with high laccase activity
One hundred foutry colonies were screened from cultures in M9 medium supplemented with 0.2mmol/l Cu2+. After secondary screening, 46 bacterial strains were picked out by color development reaction to syringaldazine. One of the potential strains showing a high level of laccase activity was named ETL-1979 and selected for further studies. The strain, ETL-1979, that formed pinkish colonies on LB agar plate, was a gram-positive, spore forming, rod shaped, 1 - 2μm long, motile bacterium, and formed white colonies on LB agar supplemented 0.2 mmol/l Cu2+. The optimum pH was 7.0 and the optimum temperature was observed at 25˚C, though they were able to grow at temperatures ranging from 20 - 55˚C. The obtained 16S rDNA PCR product was about 1.5 kb in length (Figure 1). The morphological, physiological, biochemical charac-teristics (Table 1) and the comparative analysis of DNA sequence with available database showed that WD23 was close to the members of B. subtilis. The highest sequence similarity (100%) and phylogeny based on Clustal X indicated that the strain ETL-1979 was B. subtilis (Figure 2).
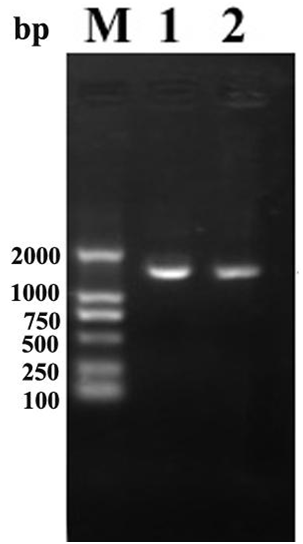
Figure 1 PCR product of 16S RDNA of B. subtillis ETL-1979. Lane M: Molecular weight marker (DL2000), lanes 1 & 2: B.subtillis strain.
Characteristics |
B. Subtillis ETL-1979 |
Colony diameter |
1-3 mm |
Colony color |
white |
Cell morphology |
Rod |
Motility |
+ |
Gelatin hydrolysis |
+ |
Urase |
+ |
Lipase |
- |
Oxidase |
+ |
Catalase |
+ |
Casein protease |
+ |
Amylase |
+ |
NO3 reduction to NO2- |
+ |
M-R reaction |
- |
V-P reaction |
+ |
Utilization of: |
|
Marmite |
+ |
Phaseomannite |
+ |
Sorbierite |
+ |
L-rnamnose |
- |
Melibiose |
+ |
Lactose |
- |
Glucose |
+ |
Maltose |
+ |
Xylose |
- |
Sucrose |
+ |
Gum sugar |
- |
Fructose |
+ |
Table 1 The morphological and Biochemical characterisitics of B.subtillis ETL-1979
Effect of metals and saline solution on bacterial growth
Metal cations Zn2+, Fe3+, Ca2+, Mn2+, Mg+ and Cu2+ (200μg/ml) all showed a certain extent of inhibition to the growth of the strain. Among them all, Zn2+ showed the highest degree of inhibition. The strain, ETL-1979, showed strength of tolerance to 10% NaCl.
The relationship between sporulation rate and laccase activity
The positive correlation of laccase activity and sporulation percentage was observed during the 10 day’s cultivation as shown in Figure 3. The result demonstrated that the laccase activity was derived from the spores.
Effect of pH and temperature on the activity and stability of spore laccase
The optimum pH of the spore laccase activity was 6.8 and the optimum temperature was observed at 60˚C. The spore laccase exhibited a higher stability in high tempe-rature and alkaline conditions than most fungal laccases. The temperature half-life of the laccase was 2.5 h at 80˚C. The pH half life time of the spore laccase was 15 days at pH 9.0.
Efficiency of dye decolorization
In order to prove the potential application of this bacterium to the treatment of wastewater containing dyestuff, the spore laccase was used for the decolorization of RBBR, alizarin red, congo red, methyl orange and methyl violet. The decolorization rates were 90% in the treatments of RBBR and alizarin red, and 50 - 70% in the treatments of other dyes (Figure 4). These results indicated that the spore laccase could decolorize most dyes efficiently without additional redox mediators.
In the present study, a novel B. subtilisstrain ETL-1979 was isolated from industrial effluent treatment plant’s soil. This strain was unable to utilize xylose and gum sugar, while the classical strain of B. subtilisdoes according to Bergey`s manual. Unlike other B. subtilisstrains conserved in our laboratory which show little laccase activity, the strain ETL-1979 exhibits high laccase activity. Therefore, strain ETL-1979 is more valuable for further research. Laccases as biocatalysts have received lots of attention because of their high capacity of oxidizing phenolic and other aromatic compounds. This advantage makes laccases suitable for some biotechnological applications, such as biodegradation of xenobiotic compounds including methoxyphenols, anilines, and benzenethiols.20,21 In contrast to fungal laccases, bacterial laccases are highly active and much more stable at high temperatures and high pH values. As stated above, most wastewaters from textile industries are characterized by a neutral to alkaline pH (around 7 - 11).22,23 For many industrial applications, it is necessary that the catalysts such as laccases are kept active in the whole process via immobilization or membrane reactors.18 Spore laccase of B. subtilisETL-1979 has a high thermostability and a high stability at alkaline conditions. These characteristics of B. subtilisETL-1979 may be of significant importance for biotechnological applications. Copper ions were added to the medium during enrich-ment culture. It is well known that copper ions are toxic even at low concentrations to lots of bacteria. However some bacterial laccases, such as CueO and PcoA play a role in copper tolerance.24 The regulation of copper homeostasis of E. coli has been analyzed, although the mechanism is still unclear.25 The main mechanism of CueO in copper resistance has been postulated to be the oxidation of the Cu+ to Cu2+.26 This process is effective for copper resistance because the Cu+ is more harmful than Cu2+.27 The present study reports that copper resistance of spore protein. B. subtilisETL-1979 can survive in copper-containing medium. However, the strain ETL-1979 is unable to form melanin-like pigment in the medium containing copper ions though CotA of B. subtiliswas associated with the formation of a brownish pigment.28,29 The strain, ETL-1979, also showed a strong endurance to high concentrations of NaCl; it can survive in 10% NaCl. This advantage makes it potentially useful to deal with wastewater containing saline solution and to reduce the time of pretreatment. To date, bacterial laccases have only been found in A. lipoferum, Alteromonas sp. MMB-1, Pseudomonas sp. KU03, E. coli, and a few species of Streptomycesand Bacillus. There is limited information on methods of enrichment culture and sampling bacterial laccases from soil of industrial effluent treatment plant. In this study, soil samples were collected from the industrial effluent treatment plant. Other reports on the isolation of bacterial species showing laccase activity was carried out from rice rhizosphere,9 seawater,20 river sludge or top-soil containing organic litter,13 soil contaminated with dye and textile industry effluent and lignocellulosic wastes.30 In our study, the spore laccase was used for the decolorization of anthraquinone and azo dyes without nutrition or redox mediators. Our results indicated that the spore laccase could decolorize the dyes efficiently within 5 days. This result is similar to that of the spore laccase from BacillusSF.4 However, few spore laccases could be reused, because it is difficult to separate spore laccase from the decolorized solution. The limitation reduces their utilization rate. Immobilized enzyme has high efficiency in dye decolorization, because immobilization can improve the utilization rate of enzyme despite the reducing enzyme activity. Our next efforts are to immobilize the spore laccases. The results demonstrate that spore laccase has potential application in dyestuff treatment. In summary, B. subtilisETL-1979 exhibiting laccase activity was screened from soil of industrial effluent treatment plnat and is well characterized. The strain showed activity of catalyzing canonical laccase substrates, syringaldazine, good growth state at 55˚C, and its spore laccase could decolorize the most regular dyes efficiently without additional redox mediators.
None.
The author declares no conflict of interest.

©2017 Shah. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.