Journal of
eISSN: 2469 - 2786


Short Communication Volume 4 Issue 1
1Instituto de Microbiolog
2Laboratorio de Bacteriolog
3Unidad Microbiolog
4Department of Biological Science, California State University Fullerton, USA
Correspondence: María Soledad Ramírez, Assistant Professor, Department of Biological Science California State University Fullerton, Fullerton, CA, USA
Received: December 26, 2016 | Published: January 26, 2017
Citation: Montaña S, Almuzara M, Pennini M, et al. IS CR2 and IS 26: two insertion sequences highly dispersed among Acinetobacter spp. clinical strains. J Bacteriol Mycol Open Access. 2017;4(1):33-36. DOI: 10.15406/jbmoa.2017.04.00082
The aim of this work is to study the dispersion of two ISs, ISCR2 and IS26, which are known to contribute in the acquisition of resistance determinants and genome plasticity, among a collection of hundred and seventy-four Acinetobacter spp. clinical isolates. PCR amplification reactions using total DNA were performed to search ISCR2, IS26, and different antibiotic resistance genes (tet(B), aphA6, sul2, floR, dfr9) previously described in the genetic context of ISCR2.
Among A. baumannii, positive amplification for ISCR2 was obtained in 66% of the included isolates and most of them (93%) were positive for IS26 amplification. The platform tet(B)::ISCR2 was found in 57% of the ISCR2 positive isolates and only 14 gave negative amplification for tet(B). In these isolates positive amplifications of genes that were previously described associated to ISCR2 -such as, aphA6, sul2, floR, and dfr9- were observed. When searching these ISs in the non-baumannii Acinetobacter studied-collection, two isolates were positive for ISCR2 and two isolates for IS26. No positive results were obtained for tet(B), but the presence of sul2, floR and dfr9 was found. Our results exposed a great dispersion of ISCR2 as well as IS26 among Acinetobacter spp. clinical isolates reinforcing the idea that the ISs play a crucial role in the plasticity and evolution towards drug resistance in this genus.
Keywords: Acinetobacter spp., ISCR2, IS26, insertion sequences, minimum inhibitory concentration
Acinetobacter are gram-negative, coccobacilli, non-fermenting aerobes and can cause a variety of nosocomial infections.1-4 Currently forty-one distinctive Acinetobacter species with unique names and characteristics were described [3, (http://www.bacterio.net/a/acinetobacter.html)]. Among this genus, A. baumannii is responsible for the majority of nosocomial infections and has an intrinsic ability to acquire and to develop antibiotic resistance determinants to all available antibiotics to treat it.5 Patient outcomes associated with extensively drug-resistant A. baumannii infections can be deadly. The CDC reports 500 deaths annually due to infections with multidrug-resistant Acinetobacter (CDC). Recently due to advances in technologies used to identify bacterial species, other members of the Acinetobacter genus have been recovered in the clinical setting and have demonstrated resistance to different antibiotics.3,6
Insertion sequences (ISs) are important for the acquisition and dissemination of antimicrobial resistance determinants, and can also contribute to resistance by insertional inactivation of proteins, such as transcriptional regulators or outer membrane protein.6,7 More than 30 different types of ISs have been reported in Acinetobacter spp.8 supporting the idea that ISs play a crucial role in the evolution of this genus and that they have contributed to the development of the multidrug-resistant phenotype observed in this genus.
Among the ISs described in A. baumannii, most of the studies referred to ISAba1, ISAba125 and ISAba3 since they are associated with blaOXA-like carbapenemases.7 Among other ISs that have been not deeply studied, we found ISCR2. ISCR are recognized as powerful elements that can capture and mobilize antibiotic resistance genes, as well as, array extended clusters of antibiotic resistance genes on plasmids or on chromosomes.9 Twenty-one members of the ISCR family have been described10 and most of them are related with antibiotic resistance.11 ISCR2 constitutes the second group of ISCR elements and it has been found in many species such as Vibrio cholerae, Shigella flexneri, Salmonella enterica, Escherichia coli, Klebsiella pneumoniae, Aeromonas salmonicida, Pasteurella piscicida. ISCR2 have been described on numerous plasmids carrying trimethoprim, tetracycline, chloramphenicol and sulphonamides resistance gene9,12 and also within resistance island.13-17
Another important IS is IS26, which was recognized to play an important role in the acquisition and dissemination of genes that confer resistance to many different classes of antibiotics. It has been reported that IS26 plays a relevant role in the genomic plasticity observed in A. baumannii. They are involved in building transposons carrying different resistance genes in the fusion of additional transposons into resistance regions and also as a contributor of the variability observed among the genomic resistance island.18
While conducting a surveillance exploring the dispersion of tetracycline resistance determinants among extensively-drug resistance (XDR) A. baumannii strains, we found a high prevalence of the genetic platform tet(B)::ISCR2 among minocycline resistant strains.19 ISCR2 has been also described in Acinetobacter spp. associated with blaVEB1, suggesting its involvement in the acquisition and the mobilization of a β-lactamase.1 Moreover, it was found as part of transposon and in AbaR-like resistant island close to tetracycline and aminoglycosides resistance genes.13-15 The aim of the present work was to further explore the dispersion of this particular insertion sequence (ISCR2) among a collection of hundred and seventy-five XDR Acinetobacter spp. clinical isolates in order to expose its prevalence and its potential role as an important IS among XDR isolates. Moreover, we also explore the presence IS26 since is known to have an important role in the plasticity of this species.
A total of 174 Acinetobacter spp. isolates were recovered from a variety of clinical sites and samples from individual patients including blood, urine, and respiratory tract, among others, from 12 different hospitals during 2010-2014. One hundred and sixty-four were A. baumannii, while the remaining ten were a novel species, Acinetobacter spp. A47 strain,2 A. pitti, A. radioresistans, A. johnsonii, A. ursingii, A. guilloiae. A. lwoffi, A. haemolyticus, A. soli and A. junii.
The species of the isolates were confirmed by MALDI-TOF MS (Bruker Daltonik, Bremen, Germany) and rpoB amplification and sequencing. The resistance profiles of the isolates to ampicilin, ampicilin/sulbactam, piperacillin/tazobactam, cefalotin, cefoxitin, cefotaxime, ceftazidime, cefepime, imipenem, meropenem, amikacin, gentamicin, nalidixic acid, ciprofloxacin, nitrofuratoin, colistin and trimetroprim/sulfamethoxazole were determined by disk diffusion according to Clinical Laboratory Standards Institute (CLSI) guidelines or using the VITEK 2 System (bioMerieux, Marcy, L’Etoile, France) employing the panel AST-082 (GNS susceptibility card) and the minimum inhibitory concentration (MIC) results were interpreted using the CLSI categories.
We extracted total DNA and used it to perform PCR amplification reactions according to the manufacturer’s instructions (Promega, Madison, Wisconsin). Specific primers for ISCR2 (ISCR2F: AAGAATTTCTCCAATGCGGG and ISCR2R: GCGGCTCCTTTTCCGACAAC) and IS26 (IS26F: GCTGGCTGAACGCGGAG and IS26R: ATACCTTTGATGGTGGC) were used. Moreover, we searched different antibiotic resistance genes (tet(B), aphA6, sul2, floR, dfr9) previously described in the genetic context of ISCR2 and we confirm its association in the required cases.9
To confirm the association of antibiotic resistance genes (tet(B), aphA6, sul2, floR, dfr9) with ISCR2 several PCR products were sequenced after purifying the DNA by using the Wizard SV Gel and PCR clean-up System kit according to the manufacturer's directions (Promega, USA). Sequencing was performed on both DNA strands, using an ABI Prism 3100 Bio Analyzer equipment. The nucleotide sequences were analyzed using the Blast V2.0 software (http://www.ncbi.nlm.nih.gov/BLAST/).
All A. baumannii isolates were categorized as extreme drug-resistant (XDR) according to Magiorakos et al.20 being resistant to carbapenems and all antibiotics tested except colistin, and in some cases minocycline. Among non-baumannii Acinetobacter isolates, six were categorized as multi drug-resistant (MDR), and three were susceptible to all antibiotics tested (Table 1).
Isolate |
MIC (mg/L)˚ |
||||||||||||||||
AMP |
SAM |
TZP |
CEP |
FOX |
CTX |
CAZ |
CEF |
IPM |
MEM |
AMK |
GEN |
NAL |
CIP |
NIT |
COL |
SXT |
|
47 |
≤2 |
≤2 |
≤4 |
≥64 |
≤4 |
≤1 |
≤1 |
≤1 |
≤1 |
≤0.25 |
≤2 |
≤1 |
4 |
≤0.25 |
≥512 |
≤0.5 |
≤20 |
23 |
≥32 |
≤2 |
16 |
≥64 |
ND |
8 |
4 |
2 |
4 |
≥16 |
16 |
≤1 |
≤2 |
≤0.25 |
≥512 |
≤0.5 |
160 |
350 |
16 |
≤2 |
≥128 |
≥64 |
ND |
32 |
≥64 |
8 |
≤0.25 |
0.5 |
≤2 |
≤1 |
16 |
≤0.25 |
≥512 |
≤0.5 |
≤20 |
306 |
≤2 |
≤2 |
≤4 |
≤2 |
≤4 |
≤1 |
≤1 |
≤1 |
≤1 |
≤0.25 |
≤2 |
≤1 |
8 |
≤0.25 |
32 |
2 |
≤20 |
7 |
8 |
≤2 |
8 |
≥64 |
ND |
8 |
8 |
2 |
≤0.25 |
≤0.25 |
≤2 |
≤1 |
4 |
≤0.25 |
256 |
≤0.5 |
≤20 |
181 |
>16 |
>16/8 |
>64/4 |
ND |
>16 |
ND |
>16 |
>16 |
>8 |
>8 |
≤8 |
≤2 |
≤8 |
≤0.125 |
>128 |
≤1 |
1/19 |
407 |
>16 |
≤4/2 |
≤4/4 |
ND |
>16 |
ND |
16 |
2 |
≤1 |
≤1 |
≤8 |
≤2 |
≤8 |
0.5 |
>128 |
≤1 |
≤0.5/9.5 |
432 |
≤4 |
≤4/2 |
≤4/4 |
ND |
8 |
ND |
8 |
≤1 |
≤1 |
≤1 |
≤8 |
≤2 |
≤8 |
≤0.125 |
>128 |
≤1 |
1/19 |
476 |
≤4 |
≤4/2 |
≤4/4 |
ND |
>16 |
ND |
4 |
4 |
≤1 |
≤1 |
≤8 |
≤2 |
≤8 |
≤0.125 |
>128 |
≤1 |
>2/38 |
Table 1 Minimum inhibitory concentrations (MICs) of antimicrobial agents in non-baumannii Acinetobacter isolates.
°Bold indicates resistance
AMP: Ampicilin; SAM: Ampicilin/Sulbactam; TZP: Piperacillin/Tazobactam; CEP: Cefalotin; FOX: Cefoxitin; CTX: Cefotaxime; CAZ: Ceftazidime; CEF: Cefepime; IPM: Imipenem; MEM: Meropenem; AMK: Amikacin; GEN: Gentamicin; NAL: Nalidixic Acid; CIP: Ciprofloxacin; NIT: Nitrofuratoin; COL: Colistin; SXT: trimetroprim/sulfamethoxazole; ND, no determinated
Surprisingly, in the A. baumannii isolates we obtained positive results for the amplification of ISCR2 in 108 out of 164 (66%). The distribution of IS26 was also high among our isolates since 93% (153/164) of the isolates resulted positive for the amplification of this IS. This last result confirms the previous suggested role of IS26 as a ubiquitous and highly disperse IS.21 Among the 164 A. baumannii isolates included in this study 102 of the isolates were harboring both IS. As we previously found a high association of ISCR2 with tet(B) in our A. baumannii population, we decided to investigate the presence of the previously reported platform tet(B)::ISCR2.19 We obtained positive results for the association of tet(B) with the ISCR2 element in 94 A. baumannii isolates (Figure 1). Moreover, 14 of the isolates that were positive for ISCR2 and IS26 gave negative amplification for tet(B). In these 14 isolates we investigated for the presence of other genes (aphA6, sul2, floR, dfr9) that were previously described associated to ISCR2. We found that all this isolates were positive for sul2, and in most (13/14) of the cases other genes were also present. We found that four isolates were positive for sul2 and dfr9, five were positive for floR apart of sul2, one was positive for aphA6, sul2 and dfr9 and another was positive for floR, sul2 and dfr9 (Figure 1). We were able to determine the association of ISCR2 with genes different than tet(B) only in one isolate, we were able to link ISCR2 with floR. Moreover, among ISCR2 negative isolates, we found three tet(B) positive isolates that were also IS26 negative.
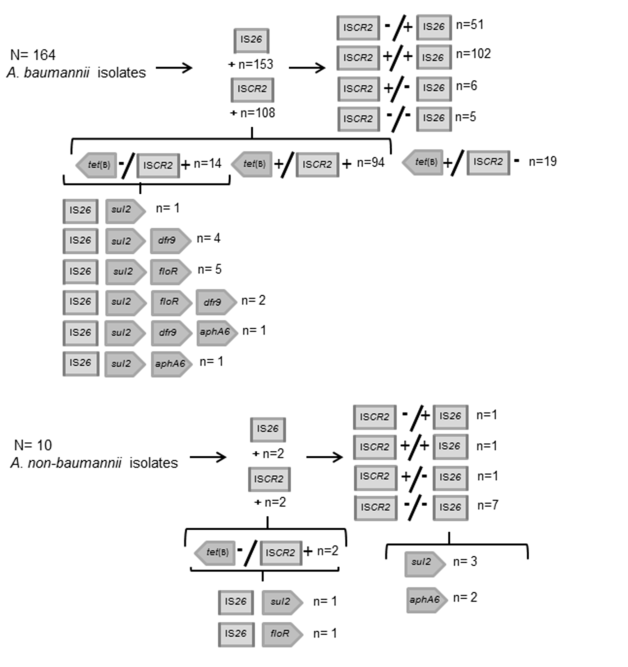
Figure 1 Diagram showing the dispersion of ISCR2, IS26and the most prevalent genes associated to ISCR2 among A. baumannii and A. non baumannii isolates.
On the other hand, in the non-baumannii Acinetobacter isolates we obtained positives results for ISCR2 in two isolates, one of which was positive for sul2 and IS26 while the other was positive for sul2 and floR. Positives results for IS26 were found in two isolates, for sul2 in five isolates, for aphA6 in two isolates, and for floR in 1 isolate. No positive results were found for tet(B).
This study shows a great dispersion of ISCR2 as well as IS26 among Acinetobacter spp. clinical isolates reinforcing the idea that ISs plays a crucial role in the plasticity and evolution towards drug resistance in this genus. The association of ISCR2 with tet(B) is the predominant platform among the A. baumannii isolates studied. However, ISCR2 is also present in other Acinetobacter spp. isolates where tet(B) is not present. We plan to perform further studies to find out the genetic environment of the ISCR2 positive isolates where tet(B) is not present. The high prevalence of ISCR2 and IS26 in our Acinetobacter spp. isolates allow us to suggest that this element is successfully been maintained is this species, giving a tool that could be involved in the acquisition and dissemination of resistance traits.
M.S.R. and D.C are members of the career investigator of CONICET, Argentina. S.M. has a Doctoral Fellowship from CONICET. This study was supported by grants UBACyT and PICT 0120 to MSR, Buenos Aires, Argentina.
The author declares no conflict of interest.

©2017 Montaña, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.