Journal of
eISSN: 2469 - 2786


Research Article Volume 4 Issue 5
1School of Medicine, Jiangsu University, China
2Jingjiang College of Jiangsu University, China
3National Institute of Parasitic Diseases, China
Correspondence: Shengxia Chen, School of Medicine, Jiangsu University, Zhenjiang 212013, China, Tel +86 13952815350
Received: April 25, 2017 | Published: May 16, 2017
Citation: Wu L, Shen J, Tang C, et al. Increased expression of toxoplasma gondii gra1 suppresses host cell apoptosis. J Bacteriol Mycol Open Access. 2017;4(5):170-173. DOI: 10.15406/jbmoa.2017.04.00110
Suppression of host cell apoptosis enable Toxoplasma gondii to proliferate, but the mechanism is not well understood. We explore the relationship between the expression of T. gondii dense granule protein 1 (GRA1) and host HeLa cell apoptosis. We inserted the T. gondii gra1 coding gene into a pET32a vector and produced recombinant GRA1 (rGRA1) in E. coli Rosetta strain. We then immunized rabbits with rGRA1 to produce anti-GRA1 serum and infected HeLa cells with T. gondii tachyzoites. We determined the expression of T. gondii GRA1 by Western blotting with anti-GRA1 serum and determined apoptosis of HeLa cells via the Annexin V-FITC/PI method. All the experiments were repeated for three times in the same condition. The expression of GRA1 decreased gradually after T. gondii infection. The rate of HeLa cell apoptosis increased more rapidly in the infected cells than in the uninfected cells. Our results suggest that the suppression of host cell apoptosis is related to the expression of T. gondii GRA1 expression.
Keywords: toxoplasma gondii, GRA1, apoptosis, ROPs, GRAs, STAT
Toxoplasma gondii is an obligate intracellular protozoon parasite. In immunocompromised hosts, such as AIDS patients, it becomes an opportunistic pathogen causing severe toxoplasmosis.1 Apoptosis is a common biological phenomenon that removes damaged or unwanted cells during development, ensuring tissue homeostasis in multi-cellular organisms.2 Cell apoptosis also plays a major role in innate and adaptive immune defense by controlling and eradicating invasive pathogens.3 T. gondii, however, can actively invade any nucleated host cell and, by down-regulating apoptosis in the cell, can evaded elimination by the host’s defense system.4 Although the mechanism of this down-regulation is not yet understood, it is known that T. gondii secreted proteins including rhoptries (ROPs) and dense granule proteins (GRAs) play an important role in this regulation.5 Many studies have examined the function of ROPs, but few have studies the function of GRAs.6 In this study, we investigate variation in the expression of GRA1 and in the rate of cell apoptosis during T. gondii invasion into HeLa cells.
Ethics Statement
All animal experiment in the manuscript were reviewed in advance by the Laboratory Animal Management Committee of Jiangsu University and also met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Cell culture and T. gondii
The HeLa cell line was stored in liquid nitrogen in our laboratory and cultured in DMEM medium (HyClone, Argentina) and incubated with 10% fetal bovine serum at 37˚C and at 5% CO2 (Tianhang Biological Technology Co., Ltd, China). The cells were stripped from the cell culture flask with a 0.25% trypsin (GBICO, USA) and 0.02% EDTA-Na2 solution. The passage operation was repeated every 2-3 days. T. gondii RH strain tachyzoites were maintained in HeLa cells. Before infection with T. gondii tachyzoites for 12 h, the HeLa cell medium was changed to a DMEM medium with 2% fatal bovine serum. The T. gondii RH strain tachyzoites were added into the culture medium and this was changed to new fresh DMEM medium (with 2% fetal bovine serum) 4hours after infection. After HeLa cell lysis, the tachyzoites were collected by centrifugation and purified using a 3-mm filter membrane.7
Cloning of the GRA1 gene and anti-GRA1 serum production
The purified tachyzoites were used for total RNA extraction performed using the RNaEXTM Total RNA Isolation Kit according to the manufacturer’s instructions (Generay Biotech Co., Ltd. China). The GRA1 gene used for His-GRA1 recombinant protein production was amplified from cDNA by PCR assay. The GRA1 primers 5’- CGGATCCCCGAAGGCGGCGACAACCAG-3’ (sense) and 5’- CGGAATTCTACTCTCTCTCTCCTGTTAGGAACCCAATGTC-3’ (antisense) yielded a 495-nucleotide product specific to the GRA1 coding gene (GenBank No. HM067753.1). The PCR product was digested with BamHI and EcoRI, ligated into a pET32a vector using T4 DNA ligase (TAKARA, Dalian, China), and transformed into an E. coli Rosetta strain. The right directional clone was confirmed by sequencing. The His-GRA1 recombinant protein was expressed in E. coli Rosetta strain that had been induced at 30˚C for 10 h with 0.4mM isopropyl-β-D-thiogalactopyranoside (IPTG). The bacterial pellets were re-suspended in lysis buffer (Tris-HCl 10mM [pH 8.0], NaH2PO4 100 mM, Urea 8 M). Cellular debris was removed by centrifugation at 16,000 g for 30 min. The cell-free extract was used for rHis-ACP purification by Ni-NTA Agarose (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. This His-ACP recombinant protein was identified by SDS-polyacrylamide gel electrophoresis staining with Coomassie blue.
The specific anti-serum of GRA1 was obtained from New Zealand rabbits (male, 3 kg, Laboratory Animal center of Jiangsu University) by three subcutaneous immunizations. The first injection comprised 1mg of recombinant GRA1 in Freund’s complete adjuvant, with two further injections also with Freund’s incomplete adjuvant administered at three week intervals. The rabbit was then bled and the specific anti-serum collected for the Western blotting assay.
Western blotting assay
Freshly released tachyzoites were boiled in SDS-PAGE sample buffer and separated on 12% polyacrylamide gels utilizing the Laemmli discontinuous buffer system.8 Proteins were transferred to nitrocellulose membranes (BOSTER, China), and the nitrocellulose strips were saturated for 1h in 5% non-fat milk in 15mM Tris-HCl (pH 8.0), 150 mM NaCl, and 0.05% Tween 20 (TNT). They were then incubated with anti-GRA1 serum diluted 1: 2,000 in TNT for 1h. After washing, the strips were incubated with goat anti-rabbit IgG conjugated to HRP (BOSTER, China) and detected using the ECL (BOSTER, China) plus Western blotting system.
T. gondii GRA1 expression
The parasites (5×106/well) were added to confluent HeLa cell monolayers in a 6-well plate (ratio of cells to parasites equals one). After infection for 1 h, all the infected HeLa cells were completely washed with PBS to eliminate parasites that had not invaded cells. We used anti-GRA1 serum and anti-T. gondii actin mAb (in mouse ascitic fluids, obtained from Professor Dominique of the University of Geneva, Switzerland) to determine the expression of GRA1 during infection.
Analysis of apoptosis
The parasites (5×106 per well) were added to confluent HeLa cell monolayers in a 6-well plate (at a cell to parasite ratio equal to one). One hour after infection, all infected HeLa cells were completely washed with PBS to eliminate parasites that had not invaded cells. After cell detachment, HeLa cells were stained with Annexin V using the Annexin V-FITC/PI kit (Vazyme, Nanjing, China). Apoptosis cells were identified and quantified by flow cytometry (FACSCalibur, BD Company, USA). Briefly, HeLa cells (1×106 per well) were washed in PBS and incubated with 1× binding buffer, propidium iodide (PI) and Annexin V-FITC for 30min at room temperature. The apoptotic cells were analyzed on a fluorescence-activated cell sorter.
Statistical analyses
Statistical analyses were performed in SPSS. Student’s t tests were performed under the assumption of equal variance and using a two-tailed test, where P≤0.05 was considered significant.
Expression of rGRA1 and anti-GRA1 serum production
Recombinant plasmids containing GRA1 gene were confirmed by BamHI/EcoRI restriction enzyme analysis. The band corresponding to the 495bp product was visualized on a 1.5% of agarose gel (Figure 1). As shown in the SDS-PAGE gel, the rGRA1 expression was induced by IPTG and its molecular weight was about 45 kDa. This is consistent with the expected molecular mass given that the expression vector produced a recombinant protein fused to a 20 kDa His-thioredoxin tag (Figure 2). In the Western blotting assay, the GRA1 (25 kDa) in tachyzoite lysis could be recognized by the rabbit anti-GRA1 serum, while the rabbit anti-GRA1 serum did not react with the HeLa cell lysis (Figure 3).
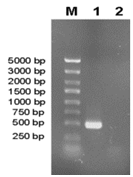
Figure 1 The GRA1 gene product amplified by RT-PCR from T. gondii (RH strain).
M: Molecular weight marker; Lane 1: GRA1 gene; Lane 2: Negative control.
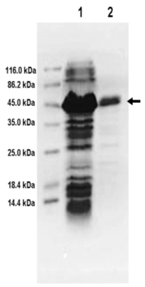
Figure 2 SDS-PAGE analysis of rGRA1 expressed in E. coli.
The E. coli lysates were electrophoresed on 12% SDS-PAGE and stained with Coomassie brilliant blue R-250. M: molecular weight marker; Lane 1: IPTG-induced E. coli with pET-32a; Lane 2: Purified rGRA1. The rGRA1 fused with His-thioredoxin tag (45 kDa) is shown with an arrow.
GRA1 expresion in T. gondii infection
The expression of GRA1 decreased gradually following the infection of HeLa cells by T. gondii. One hour post-infection the expression of GRA1 peaked and then decreased rapidly to its lowest level at 36 post-infection (Figure 4).

Figure 4 Western blotting analysis of T. gondii GRA1 expression over 36 h.
Actin: T. gondii actin expression; GRA1: T. gondii GRA1 expression.
HeLa cell apoptosis
The rate of HeLa cell apoptosis increased with time for both infected and uninfected cells, but did so more rapidly in the uninfected group. At eight hours post-infection, there was no significant difference in apoptosis rates between infected and uninfected individuals. However, by 12 hours post-infection, the rate of apoptosis of uninfected cells had increased dramatically. At 36 hours post-infection, we observed the greatest difference in the rate of apoptosis in these two groups (Figure 5).
The obligate intracellular protozoan T. gondii has evolved an intimate relationship with its host that extends to the cellular and molecular levels.9 This pathogen requires an appropriate host cell environment for its replication and is able to modify its host cell functions.10 Intuitively, the active modification of host cell growth must be a complex process, as host cells are not inherently programmed to provide an environment conducive to pathogens.11,12 Host cells have evolved primary lines of defense as countermeasures to pathogen invasion, establishment, and replication.13,14 Defenses to limit pathogen growth include apoptosis, reactive oxygen and nitrogen intermediates.15 Previously, we reported that T. gondii infection suppressed apoptosis of HeLa cells for 36 hours post-infection in uninfected cells and in cells treated with actinomycin D.16 T. gondii uses a mixture of specialized parasite secreted proteins, including ROPs, GRAs and MICs to invade cells.17,18 Many of these parasite secreted proteins are anchored on the parasitophorous vacuole membrane (PVM) and can interact with the host cells directly. The kinase activity ROPs, such as ROP2,19 ROP1620 and ROP18,21 have been exhaustively studied and shown to down-regulate host cell apoptosis.22 Moreover, kinase activity ROPs can affect the activation of signal transducer and activator of transcription (STAT) signaling pathways, leading to a downstream modulation of the secretion of IL-12 in host cells.23,24
GRA1 belongs to a dense granule protein family consisting of 12 distinct polypeptides that are stored within dense granules of T. gondii.25 During cell invasion, GRA1 was exocytosed into the parasitophorous vacuole (PV) and associated with PVM. Unlike T. gondii ROPs, the role of GRAs is not yet clearly understood. Indeed, GRA1 could activate TGF-β transcription through phosphorylation and activate apoptosis in monocytes.26 The role of GRA1 in human cell apoptosis has rarely been studied. In this study, we showed that the expression of GRA1 decreased gradually 36hours post-infection, while HeLa cell apoptosis increased more rapidly in infected cells compared to uninfected cells. These findings suggest that GRA1 suppresses HeLa cell apoptosis, in a manner similar to ROPs.
We suggest that GRA1 is anchored in the PVM and along with other secreted proteins, such as ROP2, could down-regular human cell apoptosis. This would provide a longer growth period for T. gondii in host cells and also benefit the parasite by avoiding elimination of infected cells.
This work was supported by grants from the National Natural Science Foundation of China (No. 81301453), the Laboratory of Parasite and Vector Biology of China, MOPH (No. WSBKTKT201302), the China Postdoctoral Science Special Foundation (No. 2015T80518), the China Postdoctoral Science Foundation (No. 2014M561598), Jiangsu Postdoctoral Science Foundation (No. 1402171C), and the Senior Talent Studying Initial Funding of Jiangsu University (No. 13JDG023, 13JDG127).
The author declares no conflict of interest.

©2017 Wu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.