Journal of
eISSN: 2469 - 2786


Research Article Volume 4 Issue 4
1Department of Biochemistry, Manipur University, India
2Sun Yat-Sen University, China
3Department of Biochemistry, Hyderabad Central University, India
Correspondence: Tamreihao K, Department of Biochemistry, Microbial Biotechnology Research Laboratory (MBRL), Manipur University, India, Tel +91 897 400 9605
Received: January 29, 2017 | Published: April 13, 2017
Citation: Khunjamayum R, Tamreihao K, Ningthoujam DS, et al. In-vitro antimycobacterial activities of endophytic bacteria associated with medicinal plant of manipur. J Bacteriol Mycol Open Access. 2017;4(4):104-107. DOI: 10.15406/jbmoa.2017.04.00097
Endophytic bacteria isolated from indigenous medicinal plant Solanum xanthocarpum were screened for antimycobacterial activities against Mycobacterium smegmatis using MTT assays. Of 18 isolates obtained 3 showed antimycobacterial activity. The crude extract of 3 bioactive isolates were tested against attenuated Mycobacterium bovis strain and pathogenic strain of Mycobacterium tuberculosis and their IC50 values were calculated. Crude metabolites of 3 strains showed IC50 values less than 100µg/ml. The 2 bioactive strains were identified as Streptomyces sp. and other as Bulkholderia fungorum.
Keywords: solanum xanthocarpum, endophytic bacteria, antimycobacterial activities, mtt assay, mycobacterium
TB, tuberculosis; M. tuberculosis, mycobacterium tuberculosis; MDR, multi-drug resistant; XDR, extensively-drug resistant; SCN, starch casein nitrate agar; M. smegmatis, mycobacterium smegmatis; M. bovis, mycobacterium bovis; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrtrazolium bromide
Tuberculosis (TB) is a well-known infectious disease caused by Mycobacterium tuberculosis, which commonly affects the lungs. It has afflicted humans since ancient times. Death causes by TB crosses 2 million globally with another 9 million new cases each year.1 TB is responsible for more years of healthy life lost than any other infectious disease, bar AIDS and malaria.2 The major problem with treatment of TB however lies in lack of effective treatment methods. With the emergence of multi-drug resistant (MDR) and extensively-drug resistant (XDR) strains of M. tuberculosis the disease has become a serious concern.3,4 The alarming increase of MDR-TB cases, therefore, requires a urgent development ofnew, more effective and less toxic side effects with extensive, and potent activity against resistance strains and drugs that will be able to reduce the total duration of treatment.5,6
Medicinal plants with a long history of folk medicinal practices offer hope to fulfill the needs of current demands of effective cure. These traditional formulations often contain mixtures of different chemical compounds that may act either individually, additively or synergy to improve health.7 There is ample archaeological evidence indicating that medicinal plants were regularly employed by people in prehistoric times. In several ancient cultures, botanical products were ingested for curative and psychotherapeutic purposes.8 This traditional knowledge of medicinal plants has usually resulted from trial and error experiment, and often based on speculation and superstition. India is one of the oldest countries in the World with unique wealth of medicinal plants and vast traditional knowledge for use of herbal medicine in curing various diseases. Over the past decade, there has been a proliferation of literature on the antibacterial, antituberculosis, antifungal and antiviral properties of plant extracts.9 Several reports on in vitro inhibition of Mycobacterium species by medicinal plant extracts and the bioassay-guided research for antimycobacterial properties from plants have shown sign of success. However no marketable products have been identified till date have reported few metabolites viz. norditerpenoid and 12-demethylmulticauline from the roots of Salivia multicaulis with more potent activity than the first line TB drug ethambutol, and nearly as active as rifampicin under in vitro condition.
Endophytic bacteria from medicinal plants have recently generated significant interest in the search for anti-TB drugs due to their immense potential to contribute to the discovery of new bioactive compounds. Due to close biological association between endophytes and their host plant there is more potential for discovering greater number of bioactive molecules compared to epiphytes or soil related bacteria.10,11 The anti-TB produce by endophytes are likely to possess reduced cell toxicity as the bioactive compounds may not affect the eukaryotic host cell due to symbiotic relationship between endophytes and host plants. This is of significance to the medical community as potential anti-TB drugs may not adversely affect human cells.11
The current study is based on the antimycobacterial screening of endophytic bacteria associated with indigenous medicinal plant of Manipur. It also deals with the study of antimycobacterial activity by crude extracts of bioactive strains.
Isolation of endophytic bacteria
Endophytic bacteria were isolated from roots, stem, leaves and fruits of medicinal plant Solanum xanthocarpum (local name: Leipung-khanga) following the protocols of Qin et al.12 Starch Casein Nitrate Agar (SCN), Tap Water peptone Agar, Tap Water Yeast Extract, 2.5% Water Agar and Yeast Malt Agar were used as isolation medium. The purified cultures were preserved as agar slants (4˚C) and glycerol stocks (20% v/v, -20˚C) for further use.13
Primary screening for antimycobacterial activity
Endophytic isolates were subjected to a preliminary determination of mycobacterial growth arrest and toxicity by culture filtrates (described in the following section) and MTT assays14 using Mycobacterium smegmatis (mc2 155) as the indicator. Isolates showing antimycobacterial activity were selected for further experiment. All the experiments were performed in the laboratory (F-60) approved by the Institutional Biosafety Committee (N0.UH/SLS/IBSC/Review/SB-R-11 and SB-R-14) for Mycobacterial cultures by University of Hyderabad Institutional Biosafety Committee under Department of Biotechnology, Govt. of India.
Antimycobacterial activity by culture filtrates
Strains were inoculated in Starch Casien Nitrate (SCN) broth and kept incubated in a shaker (150 rpm, 30˚C, 7d). The cultures were centrifuged (10,000 rpm for 10mins) and the supernatant collected were filtered through a membrane filter (0.2µm pore size). The pathogenic strain M. smegmatis was grown till the log phase in 7H915 media and about 1x106 cfu/ml of mycobacterium were spread onto the 7H1015 plates. 100µl of the culture filtrates were then incorporated in a well (6mm) and the plates were incubated at 37˚C for 36 h. Clearing zone surrounding the well indicated antimycobacterial activity.
Extraction of crude metabolites
Extraction was done according to Kaaria et al.16 with some modifications. Bioactive isolates were allowed to grow in 6 L SCN broth (150 rpm, 30˚C, 7d). Culture broth was then centrifuged (10,000 rpm, 10 mins) and the supernatant collected were extracted two times with ethyl acetate. The organic phase was allowed to pass through a pad of anhydrous sodium sulphate and evaporated to dryness using Rotary Evaporator (Stuart, Bibbly Scientific Limited).
The extracts were used for determining the cytotoxicity assay against attenuated M.bovisBCG and pathogenic strain of M. tuberculosis H37Rv. Mycobacterium strains were allowed to grow in 7H9 media supplemented with 10% Oleic Albumin Dextrose Catalase (OADC) till the log phase and about 1×106 cfu/ml were seeded into microplate, incubated for 24-36 hours at 37˚C. Inculum size was prepared using Macfarland standards (0.1 OD600 corresponds to 1×108cfu/ml. 20µl of 5mg/ml 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltrtrazolium bromide (MTT) was added to each well and incubated for 4 h. The formazan crystals formed were dissolved in DMSO and the plates were read at 540 nm in Enzyme-linked immunosorbent assay (ELISA reader) and IC50 values were calculated.
Characterization of bioactive strains
Genomic DNA isolation was done according to Li et al.17 The 16S rRNA gene sequence was amplified using the primers 8F (5’-CAGAGTTTGATCCTGGCT-3’) and 1522R (5’-AGGAGGTGATCCAGCCGCA-3’) (IDT, USA). The primers were designed based on the 16S rRNA gene sequence of E. coli.18 The almost complete 16S rRNA gene sequence of the strain was identified using the EzTaxon-e server database19 and aligned with the 16S rRNA gene sequences of related species using CLUSTAL X version 2.1.20
Traditionally, soil-derived bacteria have been most frequently screened for bioactive compounds against M. tuberculosis. Unfortunately, the frequency of finding new bioactive compounds from normal soil-derived bacteria is declining because of the redundancy in the isolation of known bacteria and antibiotics. Alternatively, bacteria from previously unexplored or under explored environments such as medicinal plants, marine, desert and forest ecosystems are screened.21 In recent years, pathogenic microorganisms are gaining resistance against antimicrobial agents; hence the search for new, safe and more effective antimicrobial agents is an urgent need for the emerging multidrug resistance.6 Endophytes from medicinal plants have recently given significance due to their immense potential to contribute to the discovery of novel anti-TB compounds. Endophytic bacteria have been reported to possess wide spectrum activity against many pathogenic fungi and bacteria.22,23
A total of 18 putative strains were isolated from medicinally important plant of Manipur. Based on the preliminary screening for antimycobacterial activity against M. smegmatis, 3 most promising isolates viz. SxF1 (isolated from Fruit), SxF2 (Fruit) and SxL6 (Leaves) were selected for study. The culture filtrates of the 3 strains also exhibited clearing zone of inhibition against M. smegmatis.
Crude extract strains SxF1 and SxL6 showed showed good antimycobacterial activity with IC50 values of 25 and 12.5µg/ml. However, the IC50 value of SxF2 was larger than 100 (Table 1) (Figure 1). Crude extract of strains SxF1, SxF2 and SxL6 showed IC50 values of less than 100µg/ml against H37Rv. The extracts from SxF2 though effective against M. bovis BCG (IC50=22.6µg/ml) was not as effective against M. tuberculosis H37Rv (IC50=652µg/ml) (Table 1). The culture filtrates and crude metabolites extract of Streptomyces sp. has been reported to exhibit antimycobacterial activity against M. tuberculosis H37RV.6,24 Similarly, culture filtrate and crude extract of Brevibacillus laterosporus isolated from soil inhibit the growth of Mycobacterium sp..25 Penialidin C produced by endophytic fungi Penicillium sp. showed antimycobacterial activity against M. smegmatis.26 Strain SxF1 was found to be closely related to Streptomyces harbinensis (99.51%), SxL6 was closely related to Streptomyces herbinensis (99.76%). SxF2 was identified as Bulkholderia fungorum (100%). To our knowledge, this is the first report of Bulkholderia sp. having antimycobacterial activity.27-32
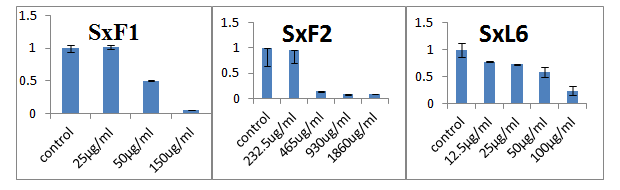
Figure 1 MTT assay for Mycobacterium smegmatis using ethyl acetate extract of culture filtrate.
Note: X axis: Amount of extracts (in mg/ ml); Y axis: Relative viability
S. No. |
Isolate |
M. Bovis (BCG) |
M. Tuberculosis (H37Rv) |
1 |
SxL6 |
83.76µg/ml |
62.48µg/ml |
2 |
SxF1 |
24.902µg/ml |
74.70µg/ml |
3 |
SxF2 |
22.6µg/ml |
652.5µg/ml |
Table 1 IC50 values of the crude extracts of bioactive strains
Of 18 endophytic bacteria obtained from medicinal plant Solanum xanthocarpum, 3 strains showed antimycobacterial activity under primary screening. Crude metabolites of the 3 strains also exhibited good antimycobacterial activity against the tested Mycobacterium sp. However, crude extract of Bulkholderia fungorum was not effective against M. tuberculosis H37Rv. The antimycobacterial compound(s) release by the bioactive strains can be used for further study for development of new, more effective and safer anti-tuberculosis inorder to control the alarming increase of MDR-TB cases in the developing country especially India.
Authors gratefully acknowledged Department of Biotechnology (DBT), Government of India (GOI) for funding DBT Twinning Programme (BT/47/NE/TBP/2010). Authors also acknowledge the grant from DBT, GOI given to the DBT-State Biotech Hub (DBT-SBTHub) (BT/04/NE/2009), Department of Biochemistry, Manipur University.
The author declares no conflict of interest.

©2017 Khunjamayum, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.