Journal of
eISSN: 2469 - 2786


Research Article Volume 9 Issue 2
Sancti Spiritus-Center for Genetic Engineering and Biotechnology, Cuba
Correspondence: Javier David Brizuela Cardoso, Sancti Spiritus-Center for Genetic Engineering and Biotechnology, Cuba
Received: June 07, 2021 | Published: June 23, 2021
Citation: Cardoso JDB, Coba IV, Torres JMF, et al. HMC Europe HG-80 autoclave qualification for its use with solid materials at the sancti spiritus (Cuba) - faculty of medicine vaccination center. J Bacteriol Mycol Open Access. 2021;9(2):99-102. DOI: 10.15406/jbmoa.2021.09.00304
The sterilization process in biotechnological industry ensures safety on materials used during drugs and biological reagents production. The most frequently used technique is sterilization by means of pressurized steam. This paper describes the HG-80 autoclave qualification and tests for steam sterilization cycle performance qualification in order to demonstrate reliability and verify compliance with sanitary regulations. In a first step, it was evaluated whether the autoclave was able to control and maintain the programmed operating temperature, by determining the temperature distribution and behavior along the chamber. In the second stage, sterilization cycles were performed in triplicate of two loading patterns where the influence of the materials on the temperature profile was studied. Simultaneously, the biological indicators challenge was executed, which play an important role in the evaluation of the process effectiveness together with the F0 concept. The F values obtained at each studied point indicate the process provides sufficient lethality to eliminate the potentially resistant forms of microbial life present in the loading pattern materials. The results obtained show that the evaluated sterilization processes generated consistent and reproducible results according to the established specifications; therefore, the validated process is considered as follows.
Keywords: sterilization process, biotechnological industry, vaccination, vaccinator, temperature, heat sterilization
The steam sterilization process is many used in biotechnological and medical productions to avoid contamination. Sterilization is achieved by exposing products to saturated steam at high temperatures (121°C to 134°C). Products are placed in a device called the autoclave and heated through pressurized steam to kill all microorganisms including spores. Unlike dry heat sterilization, steam is much more efficient in penetrating and carrying heat to every surface of the object being sterilized. Steam auto- claving is reasonably convenient, fairly efficient, and widely used for general sterilization of materials that aren’t heat, pressure, or moisture sensitive. Surgical instruments and bed linens are examples of materials that are autoclave compatible.1 When steam sterilization of medical devices, production or packaging components, final pharmaceutical product, or bulk active pharmaceutical ingredients is required, it is necessary that both the sterilization cycle(s) and the autoclave be validated. Validation of the autoclave and the sterilization cycle(s) is required by ANSI, AAMI, ISO, and the FDA to ensure that all items that are required to be sterile are able to consistently and reliably be sterilized to reduce the chance of introducing or spreading an infectious microorganism.2 ISO 17665 covers sterilization of solid as well as liquid medical devices. In some developing countries, medical devices reuse is common during vaccination interventions as well as others medical interventions, which had led to outbreaks apparition.3 Satellite, temporary, and off-site vaccination clinics play an important role in improving vaccination coverage rates and vaccinating hard-to-reach populations. In Cuba, the recent national immunization program ``Abdala´´ has reached thousands of peoples until this moment and many vaccination clinics had been adapted to achieve vaccination in many provinces. To ensure best practices in these locations is needed to follow some guidelines related to vaccine storage transport, delivery, administration, among others.4 One of these aspects is the steam sterilization assurance of many solid materials used at the immunization process. To guarantee it is needed to check both the operational and performance qualification of the autoclaves used. One of the vaccinator centers implemented is located at the Faculty of Medicine of Santi-Spiritus University and the responsibility for delivering the sterile clothing as well as other solid materials used has been left to the Sancti-Spiritus Center for Genetic Engineering and Biotechnology. The objective of this study was the HMC Europe HG-80 autoclave qualification to demonstrate the sterilization process reproducibility in the established time, and to verify that it is possible to reduce the sterilization time to 20 minutes (80% of the established sterilization time) by means of a performance and operational partial qualification of the HG-80 autoclave used for this purpose.
Autoclave features
Autoclave HG-80 (HMC-Europe, Germany): top loading, vertical, effective usable volume: 76litres, temperature max. 60°-135°, chamber dimensions (in mm): Ø364 x 730H (height), external dimension (in mm): 455W x 691 D x 1030mm H; stainless steel pressure vessel; fully automatic and changeable programs; central fast-lock with automatic FingerTip-Opening and Locking; 230 V; power 3.8 kW; capacity 3 Baskets, net weight 73kg.
Installation and operational qualification
Installation qualification (IQ) was performed by qualitative and quantitative testing of equipment components. The equipment was checked for wiring, chassis and sensors, door operation, base supports, safety valves, solenoid and shut-off valves, controls and switches, software/display, audible signals, alarms, test cycle, thermostat, isolation media, water support, vapor extraction media and equipment documentation. Operational qualification (OQ) was based on three sterilization cycles with the chamber empty. The following parameters were used: 121±0.5°C temperature, 1 atmosphere steam pressure and 25 minutes exposure time. Twelve UTT10K thermocouples (UNI-T, China) were placed and distributed to provide a representative sampling of the autoclave chamber thermal variations. The probes cover a temperature measurement range between -40ºC and 260ºC±0.75.
Performance qualification
Performance qualification (PQ) was performed for each specific load as described in Table 1. Twelve UTT10K thermocouples (UNI-T, China) were placed in the loaded chamber. A biological indicator was used for each thermocouple used for penetration temperature monitoring. For the microbiological challenge study, the biological indicator MagnaAmpTM Geobacillus stearothermophilus (Mesa Labs., USA, 2015) were placed at the coldest points of the autoclave as determined in the heat distribution study. After the sterilization process was completed, the biological indicators were incubated for 48 h at 55-60°C in a Binder IP-20 oven (Binder GmbH, Germany). Three runs were carried out in every penetration study to ensure reproducibility. BrandTM steam sterilization indicator tape was used as an adhesive, which has a chemical indicator that turns from white to black, the color change indicating that the materials have been exposed to the sterilization process. The time-equivalent cumulative lethality rate (Fphys) during the sterilization cycle was calculated to characterize the process capability using Equation 1:
|
Running characteristics |
Chamber mapping (OQ) |
Heat penetration (PQ) |
|
|
Load |
Empty chamber |
Minimal load |
Maximum load |
|
Standard cycle (121±0.5oC, 25min) |
3 |
3 |
3 |
|
Reduced cycle (121±0.5oC, 20min) |
0 |
2 |
2 |
|
Reduced cycle (121±0.5oC, 17min) |
0 |
2 |
2 |
|
Biological indicators |
No |
Yes |
Yes |
Table 1 Runnings for chamber mapping and heat penetration
Where T = 1 is the first increment of Fphys value, and X is the last time of Fphys value increment. The lethality (L) during the sterilization cycle was calculated to characterize the process capability using Equation 2:
�
The minimal required value of F0 of the process was calculated with Equation 3:
Where D121.1 is the D value at 121.1ºC of the microbiological challenge load, A is the population or concentration of the microbiological challenge load per container, B is the maximum acceptable concentration or sterility assurance level. All mathematical calculations were performed in the MATLAB software version R2015a.
The intention of autoclave qualification is to demonstrate that the sterilization procedure established in the process definition will consistently provide a sterile product. The HMC Europe HG-80 autoclave is used to sterilization of different materials used in biomanufacturing on the Sancti-Spiritus-Center for Genetic Engineering and Biotechnology, Cuba.5,6 Recently, Abdala- Vaccine intervention trials have demanded a working burden for safety assurance at Sancti-Spiritus-preventive interventions and a qualification of this autoclave for solid material as garments and other tools is required.
Installation and operational qualification
A comparison between autoclave components and those specified by the manufacturer was carried out to confirm that the equipment is correctly designed and installed for its intended use. The installation rating met the parameters specified by the manufacturer and international standards. During the autoclave operational qualification, a heat distribution study was carried out in the chamber (Figure 1). The qualification made it possible to evaluate the calibration status of the autoclave's temperature sensor with respect to the thermocouples used. The qualification of the autoclave showed that the control systems of the equipment's operating variables work within the manufacturer's specifications and international standards. The control of maximum and minimum temperatures obtained during the process and the sterilization and drying times were evaluated. It was confirmed that the equipment components are operating consistently according to the manufacturer's specifications. The thermocouples measured a highly accurate temperature respective to the sterilization time (Table 2). The lesser variability was observed on the equipment control temperature TD (VC=0.04%) and between the thermocouples, the T1 (corresponding to the four located at lower position) showed the minimal variation coefficient (VC=0.09 %). This is due to the sensors position is close to the heat inlet. On the other hands, the thermocouples used at upper and middle position showed similar variation (Table 2) and were identified four cold spots but without significance compared to the hot spots. This is due to the autoclave is small (0.08m3) and temperature variation is not a problem in almost all cases.

Figure 1 A: Chamber heat distribution compared to the displayed by the autoclave during 25min sterilization time (three runs were performed). TD: Average temperature displayed, T1: Average temperature registered by four thermocouples located at lower position. T2: Average temperature registered by four thermocouples located at middle position. T3: Average temperature registered by 4 thermocouples located at upper position. B: Theoritical F0 calculated.
|
Statistic |
TD |
T1 |
T2 |
T3 |
|
Mean |
121.52 |
121.41 |
121.27 |
121.28 |
|
± SD |
0.04 |
0.11 |
0.38 |
0.32 |
|
VC (%) |
0.04 |
0.09 |
0.32 |
0.26 |
Table 2 Heat distribution dispersion measures on chamber during the sterilization time
Performance qualification
The HMC Europe HG-80 autoclave performance qualification showed that the load sterilization process was correctly accomplished. Heat penetration into the load (max and min) and the loading effect (comparing min and max loading penetration) were studied. The sterilization microbiological efficacy was determined simultaneously with the heat penetration test (i.e., temperature probes and Biological Indicators placed adjacent to the same loads) where bioindicators were placed at the cold spots identified at the chamber mapping (Figure 2B). The microbiological challenge made it possible to evaluate the capacity of the cycle to sterilize the load under test. This was achieved by calculating the Fphys values (equivalent sterilization time at a programmed temperature for a value Z=8.5, according to the manufacturer). Geobacillus stearothermophilus bioindicators of concentration 3.0*106 spores/unit were placed. The minimum F0 value required was calculated, taking into account the record of the bioindicators. The calculation of the minimum Fo value to reduce the concentration of microorganisms to 10-6 is 23 minutes. Therefore, the Fphys values calculated for each of the sensors located adjacent to the bioindicators should be equal to or greater than a 23 minutes cumulative lethality rate. On the exposure time at approximately 121ºC, (25-minute) was possible to observe temperature uniformity, according to the sensed temperature at the different loading locations. The Fphys values of the evaluated loads were similar reaching 30min. The bioindicators located with the thermocouple revealed different color than yellow, corresponding to the non-sterilized vial (Fig. 2B). Sterility assurance level (SAL) for each load was determined. The SAL obtained in the 25-minute cycles for each load shows a population reduction in the order of 10-9.
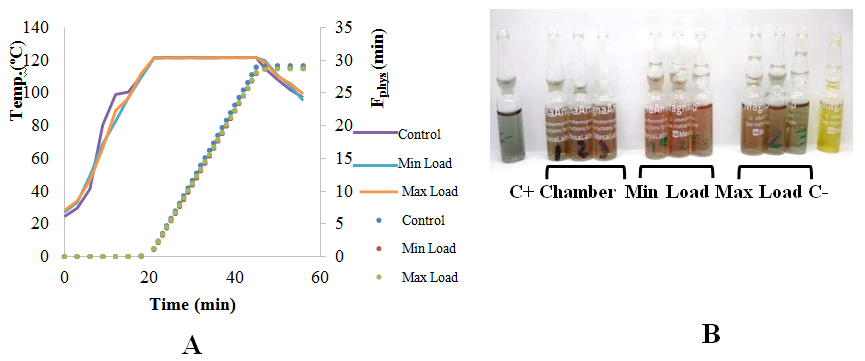
Figure 2 Heat penetration study of sterilization time of 25 min (curves and points represent the mean of three replics). A: Temperature and cumulative lethality rates. B: MagnaAmpTM Bioindicators used inside packets in all sterilizations carried out (1 bioindicator/ run). C+: Vial without Spores C-: non sterilized vial. Growth was performed following supplier specifications.
Study to reduce sterilization time in solid materials
In order to reduce the time required to achieve successful sterilization we assessed sterilization times (20 and 17 min) to verify the effect on safety assurance level (SAL) and propose a reduction of sterilization time to reduce operational costs. At the 20 min- sterilization was obtained an Fphys reaching 23 min which provides a SAL value of 2.0*10-6. Microbiological validation, which consists of tests involving bioindicators, should be performed concomitant with heat penetration studies to verify independent of the temperature data results that the minimum F0 value is met at the coldest spot of the load. For terminal moist heat sterilization, heat penetration studies must demonstrate an SAL of 10-6.7 In those terms, the 20min-running could be analyzed for posterior validation since biological indicators as well as temperature and SAL values were under standard regulations (Figure 3 & Table 3). On the other hand, at the 17 min-running, although bioindicators showed successful sterilization, the calculated Fphys did not reached to 20min, giving a SAL of 2.0*10-4. So in this sense, the established parameters were not satisfied.
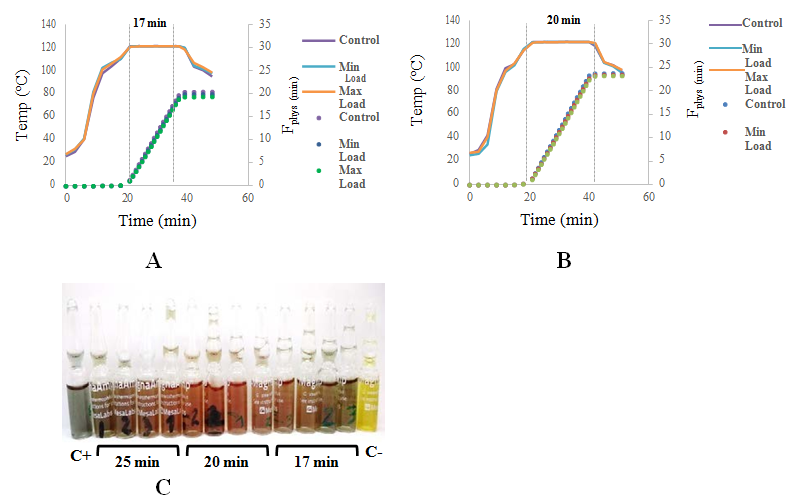
Figure 3 Heat penetration study of different sterilization times (A: 17min and B: 20min) (curves and points represent the mean of two replics respectively). A and B: Temperature and cumulative lethality rates. C: MagnaAmpTM Bioindicators used inside packets in all sterilizations carried out. The bioindicators used in the 25min sterilization was included. C+: Vial without Spores C-: non sterilized vial. Growth was performed following supplier specifications.
|
Sterilization time |
SAL |
|
20 min |
4,7*10-9 |
|
25 min |
2,0*10-6 |
|
17 min |
2.0*10-4 |
Table 3 Sterility assurance level (SAL) attained to different sterilization times
The HMC HG-80 autoclave was successfully qualified under national standards for solid materials which certify it for use in the sterilization of utensils needed in the vaccinatorium created in the Sancti Spiritus Province Faculty of Medical Sciences as well as other nearby ones.
None.
The authors declare that there is no conflict of interest.

©2021 Cardoso, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.