Journal of
eISSN: 2469 - 2786


Research Article Volume 7 Issue 4
Department of Hydraulics and Sanitation, University of São Paulo, Brazil
Correspondence: Sarah Regina Vargas, Department of Hydraulics and Sanitation, University of São Paulo, São Carlos, SP – Brazil,
Received: July 24, 2019 | Published: August 14, 2019
Citation: Vargas SR, Santos PVD, Casali SP, et al. Growth of Raphidiopsis raciborskii and Monoraphidium contortum from a subtropical Brazilian Reservoir: interaction, saxitoxin production and response to the trophic state. J Bacteriol Mycol Open Access. 2019;7(4):86-92. DOI: 10.15406/jbmoa.2019.07.00250
In order to investigate the influence of trophic state from a subtropical reservoir in the state of São Paulo, Brazil, in the competition between the species dominant, Raphidiopsis raciborskii and Monoraphidium contortum, an assay was carried out of interaction between these microorganisms simulating the reservoir in its current conditions, mesotrophic, and another in a supereutrophic condition, as well as detect the production of saxitoxin by cyanobacteria. The experiments were conducted in a climate-controlled room for 15days in triplicate for pure and mixed cultures of each species. Growth curves were developed according to the biovolume-to-biomass and were calculated the growth rates. The saxitoxin synthesis was determined by ELISA. When simulating the mesotrophic and supereutrophic environments, R. raciborskii did not show any differences in its growth and in the saxitoxin production in the interaction with M. contortum, when compared to its control. Concerning M. contortum, your growth was affected in the interactions with cyanobacteria in both trophic state, although the chlorophyceae presented an increasing specific growth rate. The reduction of the growth of M. contortum in interaction with R. raciborskii shows the increase in trophic state can increase the competitiveness of R. raciborskii in the reservoir and to favor your dominance.
Keywords: cyanobacteria, green algae, trophic states, competition, saxitoxin, biovolume
The Itupararanga Reservoir is among the most important reservoirs of ecological and economic interest in São Paulo State, as it is located in an Environmental Protection Area (APA Itupararanga) and is a water supply source for Sorocaba and its region.1 However, the basin of the Itupararanga reservoir has suffered environmental degradation due to growing urbanization and consequent irregular effluent discharge, as well as agricultural practices caused by the reduction in water quality.2,3 The increase of nutrients can contribute to the eutrophication process,4 and one of the main consequences of the change in the water quality is the proliferation of phytoplankton biomass, mainly cyanobacteria, as already observed in this reservoir.5‒7According to the Environmental Sanitation Technology Company in the State of São Paulo (CETESB) (2014), the Itupararanga Reservoir offers good quality water for public supply, although some problems with its trophic state have been observed.7 Cunha & Calijuri showed data that classifies it as mesotrophic and some points in a eutrophic condition.8 One of the most important biological communities affected by the trophic state is the phytoplankton, as it is the base of the food chain in aquatic ecosystems.4,8 Due to different forms of life, these organisms respond differently to light, temperature and nutrient regime and may occur in a wide range of environments in virtually all longitudes, latitudes and altitudes in the world.9,10
Even having different forms of life, physiological properties and different tolerances to abiotic factors, there is a coexistence of various species of phytoplankton in aquatic ecosystems. The development and change in the phytoplankton community is the result of interactions of factors such as light, temperature, concentration of inorganic nutrients and organic micronutrients, competition for resources, predation and parasitism. However, competition between one species and another is relative as the physical and biotic conditions can be altered in the environment and change the development of the species.4,8,9,11 Various research interactions between phytoplankton organisms were developed aiming to understand the competition for resource use. Rodrigo et al.,12 studied the growth characteristics of eight phytoplankton species in cultures simulating oligotrophic and eutrophic environments.12
Hyenstrand et al.13 investigated the competition between chlorophyceae and cyanobacteria in different ways of supplying inorganic nitrogen.13 Kim et al.,14 analysed the effects of limiting nutrients and the N:P ratios on the growth of phytoplankton.14 Showed the influence of limitation by phosphorus on growth, photosynthetic efficiency and morphology of Raphidiopsis raciborskii (Cyanobacteria), as it is currently called,15 formerly called Cylindrospermopsis raciborskii.16 Therefore, limiting environmental factors are important variables which determine and influence phytoplankton growth and, are consequently considered selective factors in the ecology of these organisms.17 In some periods, the Raphidiopsis raciborskii dominance (Cylindrospermopsis raciborskii) was observed in the Itupararanga Reservoir.5,6,18 The presence of R. raciborskii is a matter of concern because it is a potentially toxic species, able to produce cylindrospermopsin and saxitoxin. There is also an abundance of Monoraphidium contortum (Chlorophyceae) (Komárková-Legnerová) in the reservoir which, due to it being from different group of R. raciborskii, arouses interest in terms of researching the growth between these species in interaction.6 To clarify this problem, simulation experiments of the Itupararanga reservoir were conducted at their current trophic, mesotrophic7,8 and supereutrophic states, demonstrating the interaction between R. raciborskii and M. contortum in order to characterize the growth of these species and determine their competitive advantage. Moreover, the effect of the interaction on the production of saxitoxin by R. raciborskii was observed and may contribute to the management and conservation of this water body concerning the growth prospects of these species.
In order to carry out the experiments, firstly the samples were collected manually on the subsurface of eight sampling stations at the Itupararanga Reservoir (Figure 1) to obtain and isolate the studied species. Afterwards, the samples were mixed in the laboratory to obtain a composite sample so that there would be no influence from the sampling station. Based on the composite sample, enrichment was maintained in ASM-1 culture medium19 to isolate Monoraphidium contortum and Raphidiopsis raciborskii by successive subcultures of colonies on solid media using the exhaustion method until a pure colony was obtained.20,21 A single strain isolated from each species, R. raciborskii22 (Cr 01) and M. contortum (Mc 01) was used in this research. They are kept in the algae and cyanobacteria bank of the Biotoxicological Laboratory of Continental Waters and Effluents (São Carlos School of Engineering / University of São Paulo). Both were maintained in culture medium ASM-1, pH 7.8, 12hours light/dark, light intensity of 60µmol photons m-2s-1 of irradiation obtained with cool white fluorescent tubular lamp.

Figure 1 Location of the hydrographic basin and the Itupararanga Reservoir.22
Experimental design
An interaction experiment was carried out with R. raciborskii and M. contortum simulating the mesotrophic and supereutrophic environments of the Itupararanga Reservoir, according to the trophic state index proposed by Lamparelli23 adapted from Carlson24 the phosphorus concentration used to calculate the trophic state index of cultures medium corresponds to the orthophosphate concentration, in the form of phosphorus available for phytoplankton.9 The experiment lasted 15days and was maintained at 24°C in a climate-controlled room with a 12hours light/dark, irradiation of 60μmol photons m-2 s-1, and a pH value of 7.8. The cultures in batch was carried out in triplicate in 250mL Erlenmeyer flasks using cotton plugs, with a total volume of 100mL, that is 3 controls for each species (monoculture) and 3 for the interaction between them. Samples were aseptically collected from the Erlenmeyer flasks for density, biovolume and saxitoxin analysis. The optimum amount of inoculum of each species at the beginning of the interaction experiment was calculated based on the biovolume in the cultures.25 This measure was taken so that the biomass of the species was initially similar, because M. contortum has smaller cells than the R. raciborskii trichomes.
Nutritional condition of the experiments
The dissolved nutrient concentrations tested in the assays were based on the results obtained from the São Paulo Research Foundation (FAPESP) Thematic Project.26 The total of average concentrations obtained from the water column of nitrogen forms (nitrite, nitrate and ammonia) and dissolved phosphorus (orthophosphate) were used, as well as the average of micronutrient concentrations determined by atomic absorption spectrometry.27 According to these results, changes were made to the composition of the standard ASM-1 culture medium,19 to simulate the mesotrophic reservoir, and the concentrations were estimated for the supereutrophic condition (Table 1). To simulate the mesotrophic condition, the initial concentration of 8.8μg.L-1 and supereutrophic of 35.3μg.L-1 of orthophosphate were used, in both with 450µg.L-1 of nitrate, establishing an initial NID: PID ratio of 113:1 and 28:1, respectively.
Analysis of culture growth and saxitoxin concentrations
Aliquots of 500µL were removed from each flask of culture every two days to calculate the density28 and the cell volume of 30 individuals of each species to calculate the biovolume in a Fuchs-Rosenthal chamber.25 Afterwards, the growth curves were made by biovolume. Based on the exponential growth phase of the species, calculations of the specific growth rate (µmax) and doubling time (Dt) were also made.29 Growth curves of the species were plotted from the average of the biovolume of control cultures of each species and the interaction cultures over time. Aliquots of 2mL were taken once every three days during the experiment to determine the saxitoxin, 6 total samples, starting at day 0. The samples was stored in sealed glass jars and then frozen in a freezer at -20°C. Saxitoxin was determined using the ELISA biochemical method and the total saxitoxin concentration was extracted as described by Berry & Lind30 & Yilmaz et al.,31. According to the results obtained from the total saxitoxin concentration per sample, this unit was converted into the saxitoxin concentration by the R. raciborskii biovolume respective to its sample.
First for the analysis of the results, was performed the normality and homogeneity test. The Origin Pro 8.0 software was used, and to indicate the significant difference, p≤0.05 was adopted. A comparison was made of the growth using the Student´s t-Test between the data sets of the interaction and control of the species on each day of the analysis until the end of the assay. Statistical analyses were carried out to compare the difference of the saxitoxin concentration: between the days during the experiment, using the Analysis of Variance (ANOVA) and Tukey post-hoc test and, in addition, the Student´s t-Test was used to analyze the saxitoxin production between control and interaction in each trophic state, and to compare the saxitoxin production between mesotrophic and eutrophic conditions in the controls and interactions cultures.
The results are presented as averages of the triplicates of the controls and interaction of the species for the biotic variables, biovolume (μm3.mL-1), specific growth rate (µmax) and doubling time (Dt); and abiotic, saxitoxin concentration (µg.µm-3). The initial biovolumes for each trophic state were similar for the two species, showing no significant differences (p≥0.36 for mesotrophic and p≥0.32 for supereutrophic) (Figure 2 & Figure 3) (Table 2). In the mesotrophic condition, the M. contortum growth curves (Figure 2) of the control and interaction started to show significant differences in the biovolume from the third day (p<0.045) until the end of the experiment, and were higher in the control. The µmax of M. contortum was significantly higher in the control compared to the interaction (p=0.027). This difference was not observed in the Dt, although the probability was close to the considered significant value (0.08) (Table 3). In contrast, when comparing the growth curves in the control and interaction of R. raciborskii (Figure 2), there was no significant difference. There was only a difference on the tenth day (p=0.048) between the control and interaction for this species, remaining until the end of the assay with a similar biovolume (Table 2). There was also no significant difference in the μmax and Dt between the control and interaction for R. raciborskii (Table 3).
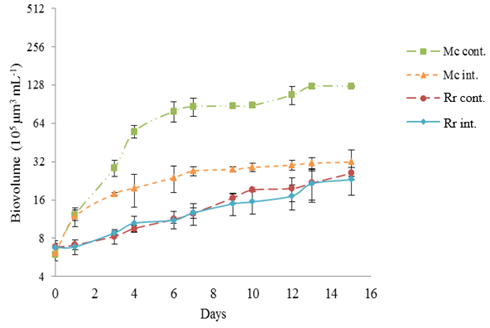
Figure 2 R raciborskii and M. contortum growth curves by day (D) based on biovolume averages (105 µm3 mL-1) in the simulation of the mesotrophic environment. Mc cont. – M. contortum control; Mc int. – M. contortum interaction; Rr cont. – R. raciborskii control; Rr int. – R. raciborskii interaction. Y axis on logarithmic scale.Vertical bars show the standard deviations (n=3).
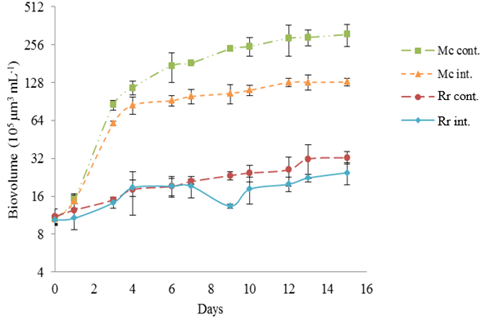
Figure 3 R raciborskii and M. contortum growth curves by day (D) based on the biovolume averages (105 µm3 mL-1) in the simulation of the super eutrophic environment. Mc cont. – M. contortum control; Mc int. – M. contortum interaction; Rr cont. – R. raciborskii control; Rr int. – R. raciborskii interaction. Y axis on logarithmic scale. Vertical bars show the standard deviations (n=3).
Simulation |
Orthophosphate (mg L-1) |
Nitrate (mg L-1) |
Micronutrients (mg L-1) |
TSI |
|
Mesotrophic |
0.0088 |
0.45 |
Zinc |
0.0875 |
55.5 |
Iron |
0.2425 |
||||
Manganese |
0.021 |
||||
Supereutrophic |
0.0353 |
Copper |
0.0135 |
63.9 |
|
Boron |
0.9 |
||||
Cobalt |
0.0075 |
||||
Table 1 Concentrations of nutrients used to simulate the mesotrophic and supereutrophic Itupararanga Reservoir, and trophic state index (TSI).23
Biomass: Biovolume (105 µm3 mL-1) |
|||||
Trophic State |
Period |
Cultures |
|||
Mc control |
Mc interaction |
Rr control |
Rr interaction |
||
Mesotrophic |
Initial |
5.93±0.23 |
6.07±0.77 |
6.79±0.52 |
6.76±0.86 |
Final |
125.29±1.25 |
31.81±7.5 |
25.92±2.96 |
23.15±5.70 |
|
Supereutrophic |
Initial |
10.59±0.86 |
10.47±0.88 |
11.03±1.61 |
10.35±0.91 |
Final |
309.4±61.26 |
129.34±9.44 |
32.45±3.74 |
24.61±4.8 |
|
Table 2 Initial and final bio volume averages of M. contortum (Mc) and R. raciborskii (Rr) (105.µm3.mL-1), with standard deviations (n=3)
Cultures: Monoraphidium contortum Control – Mc control; Monoraphidium contortum interaction– Mc interaction; Raphidiopsis raciborskii control – Rr control; Raphidiopsis raciborskii interaction – Rr interaction
Specific growth rate (µ) and doubling time (Dt) |
|||||
Parameter |
Trophic |
Culture |
|||
Mc – control |
Mc – interaction |
Rr – control |
Rr - interaction |
||
µ(day-1) |
Mesotrophic |
0.37±0.02* |
0.14±0.08 |
0.09±0.01 |
0.09±0.03 |
Supereutrophic |
0.68±0.07 |
0.58±0.07 † |
0.07±0.01 |
0.06±0.02 |
|
Dt(day) |
Mesotrophic |
1.86±0.13 |
6.12±3.2 |
7.55±0.81 |
8.90±3.64 |
Supereutrophic |
1.03±0.1 |
1.2±0.13 |
10.26±1.67 |
11.93±4.73† |
|
Table 3 Comparison of the specific growth rates (µ) and Doubling Time (Dt) (average with standard deviation, n=3) of M. contortum (Mc) and R. raciborskii (Rr)
*Difference between Mc control and Mc interaction p=0.027
†Difference between Mc interaction and Rr interaction p<0.020
In the interaction, the biovolume of R. raciborskii was smaller than M. contortum on the first day until the twelfth day, with p<0.034 for all days except on the fourth, although the statistics showed a value close to the considered significant value (0.052) (Figure 2). From the thirteenth day, the situation was reversed and the two species did not show any differences in the biovolume. Regarding the μmax and Dt, no statistical significant differences were observed (Table 3). In the simulation of the reservoir in a supereutrophic environment, the M. contortum growth curves (Figure 3) demonstrated that in the control, the biovolume increased until the end of the experiment and reached 3.09.107 μm³.mL-1. In the interaction with R. raciborskii, a maximum of 1.29.107 µm³.mL-1 was obtained (Table 2). The growth showed a difference from the third day of the experiment, remaining so until the end (p<0.04), except on the fourth day due to a higher standard deviation of the average (p=0.051). This data shows a growth inhibition of this species when interacting with R. raciborskii. Regarding µmax and Dt, no significant differences were observed between green algae cultures of control and in the interaction (Table 3).
Regarding the growth of R. raciborskii (Figure 3), the control and interaction were similar, with a significant difference only on the ninth day (p=0.007) probably due to the reduction in cell volume that occurred in the species trichomes. The data related to similar R. raciborskii growth in the control and interaction corroborate with the statistical results of the comparison concerning the μmax and Dt, as there are no significant differences of these parameters between these cyanobacteria cultures. In the interaction, the two species began the experiment with similar biovolumes and both showed an increase in biomass until the end of the experiment (Figure 3). However, M. contortum had a significantly higher biovolume compared to R. raciborskii, with a significant difference from the third day to the end of the experiment (p<0.013). There was also a difference in the µmax and Dt of the species in the interaction (Table 3).
Saxitoxin synthesis
The results shown are averages with standard deviations of the saxitoxin concentrations by biovolume (Table 4). In the mesotrophic condition, the highest and lowest saxitoxin concentrations, both in the control and interaction occurred on day 0 and the fifteenth day, respectively. In the analyzed days between the control cultures, there were significant differences of saxitoxin concentrations on day 0 and the sixth day compared to all the other days, and there were no difference between 0 and sixth day. In the interaction cultures, there were no differences between the days. Comparing the saxitoxin concentrations between the control and interaction there were no significant differences, however in both cases there was a decrease in the concentration. In the supereutrophic simulation, comparing the saxitoxin concentrations between the control and interaction there were no significant differences. In addition, from the beginning to the end of the experiment, no significant differences in the saxitoxin concentrations were found between the days when the cyanobacteria control and interaction. To analyse the differences of the saxitoxin concentrations of the R. raciborskii controls and interactions between different trophic states, we compared the results of the analysis on a daily basis. Among the R. raciborskii controls, it was observed that the saxitoxin concentration was significantly higher in the mesotrophic environment compared to the supereutrophic one on all the days. Comparing the saxitoxin concentrations of cyanobacteria in the interactions at different trophic states, it can be observed that there were differences in the toxin/biovolume relation between the mesotrophic and supereutrophic environments only on the sixth and twelfth days.
Some researches of interaction between phytoplankton organisms were carried out to investigate the competition for limiting nutrients and in different forms of availability and concentrations.13,32‒34 There are also studies that show the relationship of nutrients and micronutrients with the morphology and growth of certain cyanobacteria and microalgae.12,14,16,35,36 Zhu et al.,34 studied different concentrations and nitrogen and phosphorus relationships, simulating oligotrophy, eutrophy and hypereutrophy in pure and mixed cultures of Microcystis aeruginosa (Cyanobacteria) and Scenedesmus quadricauda (Chlorophyceae).34 They found that the best conditions for growth and reproduction for both species occurred in a eutrophic environment and, when in competition, M. aeruginosa had advantages in conditions of lower concentrations of nutrients, while S. quadricauda in higher concentrations of nutrients. The interaction between cyanobacteria and chlorophyceae was investigated by Rodrigo et al.,12 They conducted experiments using eight species of phytoplankton, including two filamentous cyanobacteria, Limnothrix redekei and Planktothrix agardhii and M. contortum (chlorophyceae). The experiments consisted of analysing the growth of these species in monoculture in oligotrophic and eutrophic conditions and the results showed that the growth and biomass of M. contortum were higher in a eutrophic environment. In contrast, L. redekei showed better results in an oligotrophic condition, both in density and biomass, and P. agardhii did not differ between the treatments.
Data from Rodrigo et al.,12 & Zhu et al.,34 corroborate this research when it is observed that M. contortum had the highest growth rate in higher concentrations of nutrients, and R. raciborskii did not show any significant difference in its growth in both simulated environments, as P. agardhii studied by Rodrigo et al.,12 Concerning the interactions carried out between cyanobacteria and chlorophyceae in the simulation in a mesotrophic environment, data showed that R. raciborskii was not inhibited by the presence of M. contortum. In contrast, the Chlorophyceae´s growth was inhibited by the cyanobacteria, as the M. contortum control in relation to the growth and the specific growth rate was significantly higher compared to this species in the interaction. Although the M. contortum growth was inhibited, there was a coexistence of this with R. raciborskii in the mesotrophic condition. On the fifteenth day, Cyanobacteria remained in the exponential phase and the stationary phase of chlorophyceae, which could be an indication that R. raciborskii has competitive advantage in a mesotrophic environment because this species growth was not affected by M. contortum. Similar to what happened in the simulation of the supereutrophic environment; it was observed that M. contortum´s growth was inhibited in the interaction with R. raciborskii compared to its control, although chlorophyceae presented a final biovolume significantly higher than that of cyanobacteria. However, when R. raciborskii in the interaction is compared to its control, it can be observed that the growth of the species was similar, and is not affected by M. contortum. Furthermore, the chlorophycea beginins firstly the stationary phase, while cyanobacteria remains in the exponential phase. This fact contributes to the competitive advantage of R. raciborskii as it continues to grow, and not have its growth inhibited.
Research on the relation of nitrogen and phosphorus concentrations demonstrates the lower ratio between this nutrients favors the proliferation of cyanobacteria.14,36‒38 This contributes to the affirmations that in more eutrophic environments, R. raciborskii is more competitively successful.
The results of this research in the supereutrophic environment, in which M. contortum has its growth inhibited in interaction with R. raciborskii, corroborated with the literature that in most eutrophic environments this cyanobacterium tends to predominate. However, this is worrying about the quality of water, since it is a species able to produce toxins.39 In addition, there are environmental reports showing the increase of cyanobacteria in the Itupararanga Reservoir.7 Despite evidence favoring the success of R. raciborskii, Reynolds40 states that on an evolutionary scale, some organisms develop morphological and physiological strategies that can improve their suitability in certain environments, although no species is well suited for all environmental conditions.40
A favorable morphology to the rapid nutrient exchange occurs in organisms with greater area / volume ratio, that is, they have smaller sizes, as is the case of M. contortum. However, larger organisms, as R. raciborskii, have advantages in nutrient storage, motility and persistence. The theory of competing for resources between the two species reported by Tilman et al.,41 shows that when these species with similar growth rates are cultured together with concentrations of limited resources, the species that obtained the highest nutrient uptake rate is able to grow more quickly than the other, as what happened with M. contortum.41 However, the species that obtains less growth can maintain its growth from another resource, even at low concentrations, which may be related to the continuous growth of R. raciborskii in both simulated environments. Concerning the saxitoxin production, in both simulated environments, the toxin / biovolume relationship decreased as cyanobacteria advanced in the exponential phase, and is coincident both in the control and interaction, the growth phases and the saxitoxin production, and there were no differences in the saxitoxin production when the species is found in the interaction with M. contortum. The causes of toxin production by cyanobacteria are not clear yet, but there is a hypothesis that these substances have protective functions against grazing, and could be related to competing for resource and growth conditions.4 However, the results of this research showed that there was no difference in the saxitoxin production during the interaction of the species in the two simulated trophic states.
The saxitoxin concentrations detected in both experiments were lower than the permitted limit in the Brazilian aquatic ecosystems. The MS Ordinance No. 2914 from the Ministry of Health recommends that saxitoxin concentrations in drinking water should not exceed 3μg L-1.42 Although the concentrations of this toxin in the simulation of the Itupararanga Reservoir were below those permitted by law, the situation is a matter of concern because it is primarily used for public supply, and the causes of toxin production are unknown. The presence of cyanotoxins in the water are recently recognized as a public health problem.43 Other species or environmental changes can influence the phytoplankton community and modify individuals which dominate this reservoir and increase the cyanotoxin production. This change in the community is a result of the interactions of various factors, such as light, temperature, pH, macronutrient concentrations and micronutrients, competition for resources, predation, hydrological cycle, hydrodynamics in reservoirs, among others.4,9 Therefore, the R. raciborskii and M. contortum populations in the Itupararanga Reservoir can be affected in their growth and dominance due to various factors. However, according to investigations, the water quality of this ecosystem is decreasing,8 and is becoming increasingly eutrophic, which can increase the competitiveness of R. raciborskii in the reservoir in a scenario of increasing dominance of this cyanobacteria.
There was evidence that R. raciborskii reduced M. contortum growth in both nutrient conditions in the interaction experiments of simulation of the Itupararanga Reservoir. Regarding the saxitoxin production, there were no significant differences between the R. raciborskii control and it in the interaction with Chlorophyceae. R. raciborskii and M. contortum coexist in supereutrophic environments. Therefore, R. raciborskii did not present inhibition in its growth by the presence of M. contortum demonstrates possible persistence of this Cyanobacteria in the reservoir and inhibition of the development of Chlorophyceae. Interaction bioassays with the predominance species of subtropical ecosystems support the understanding of influence trophic status and toxin synthesis in this environmental, contributing to water resources management and conservation of the Itupararanga Reservoir.
We would like to thank CAPES (Coordination for the Improvement of Higher Education Personnel) for the scholarship and the financial support from the thematic project entitled "Contribution to knowledge of the carbon cycle in the Itupararanga Reservoir as a subsidy for the sustainability of the Sorocaba River Basin (SP)" awarded by FAPESP (São Paulo Research Foundation) (process 2008/55636-9) to Maria do Carmo Calijuri.
Authors declare that there is no conflict of interest.
None.

©2019 Vargas, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.