Journal of
eISSN: 2469 - 2786


Research Article Volume 6 Issue 2
1Department of Physiological Sciences, Federal University of S
2Department of Animal Biology, State University of Campinas, Brazil
Correspondence: Eliton da Silva Vasconcelos, Department of Physiological Sciences, Federal University of São Carlos, SP, Brazi, Tel +55 16 3351 8968
Received: January 13, 2018 | Published: March 27, 2018
Citation: Vasconcelos ES, Salla RF. Enhancement of clavulanic acid production by mutant Streptomyces clavuligerus. J Bacteriol Mycol Open Access. 2018;6(2):122–125. DOI: 10.15406/jbmoa.2018.06.00188
Background: Clavulanic acid (CA) is a potent β-lactamase inhibitor used to combat resistance to penicillin and cephalosporin antibiotics. There are several pathogenic microorganisms that are capable of secreting β-Lactamases. These enzymes catalyze the hydrolysis of the β-lactam ring of penicillins and cephalosporins, and their hydrolysis products have no antibiotic activity. Since the first clinical applications, the efficiency of the β-lactam antibiotics has been declining, due to the astonishing increasing number of bacteria capable of displaying β-lactam resistance. Thus, the genetic production improvement using physical and chemical mutagenic agents is an important strategy in programs of industrial production of bioactive metabolites, such as CA.
Objective: The objective of the study was to enhance CA production using chemical and UV mutagenesis on Streptomyces clavuligerus.
Methods: Streptomyces clavuligerus ATCC 27064 was used as the standard strain in all the experiments. The mutant strain was first obtained by chemical mutagenesis using the agent MMS and next the same strain undergoing one more mutation was obtained by UV light. Cellular biomass was analyzed based on the dry weight method and the concentration of CA was determined by HPLC analysis.
Results: CA production increased by 28% with mutant strain when compared to the wild-type strain. The mutant strain obtained 812mg L-1 concentration in 72h of fermentation, while the wild-type strain obtained only 639.7.1mg L-1 at the same time, and the productivity at this moment was 11.28 and 8.88mg L-1 h-1 respectively. The mutant strain had a significant increase in biomass growth between 36 and 60h.
Conclusion: The study showed that random mutations can lead to the improvement in CA production in S. clavuligerus. This finding reinforces the importance of using this method for productivity improvement.
Keywords: clavulanic acid, streptomyces clavuligerus, mutation, strain improvement
β-lactam antibiotics are among the most popular classes of antibacterial agents, whose mechanism of action consists on the inhibition of bacterial cell wall synthesis.1 The production of β-lactamase is the most important mechanism for bacterial resistance to β-lactam antibiotics.2 Secondary metabolites produced by bacteria exhibit potent biological activities and have been developed extensively for antimicrobial, anticancer, and other vital therapeutic applications.3 In this sense, the CA is a secondary metabolite that was first detected in Streptomyces clavuligerus (S. clavuligerus) and is a potent inhibitor of a wide range of β-lactamases from pathogenic organisms. CA effectively inhibits the activity of β-lactamases of molecular classes A and D, such as cephalosporinases, penicillinases, and broad spectrum β-lactamases, and thus it has been widely used clinically to treat diseases caused by β-lactam resistant bacteria.4 The combined therapy of CA +β-lactam antibiotic has been very successful in preventing infections due to Gram-positive (Staphylococcus sp.) and Gram-negative (Klebsiella sp. Hemophilus sp. Proteus, Shigella, Pseudomonas) β-lactamase producing pathogens.1
Streptomyces clavuligerus ATCC 27064 (www.atcc.org) was used as the standard strain in all the experiments. This strain underwent two mutations in order to improve the production of CA. The mutant strain was first obtained by chemical mutagenesis using the agent MMS following the technique described by Stonesifer & Baltz.13 The mutant strain, when cultivated in solid medium, lacked pigmentation in their mature spores (data not shown), being classified as whi mutants (“white” - Chater14). After MMS mutation, the same strain undergoing one more mutation was obtained by UV light, as described by Lee et al.8 The wild-type strain is classified in the gray series of category IV from Streptomyces genera, based on the dark greenish pigmentation shown by its mature spores.15 All of the microorganisms were conserved as vegetative cell suspensions in cryoprotective 10% p/v glycerol stocks stored at -70°C.
Culture media
The wild-type and the mutant strains were initially cultivated in reactivation medium (composition in g.L-1 in distilled water), glycerol, 15.0; bacto-peptone, 10.0; malt extract 10.0; yeast extract 1.0; K2HPO4, 2.5; MgSO4.7H2O, 0.75; MnCl2.4H2O, 0.001; FeSO4.7H2O, 0.001; ZnSO4.7H2O, 0.001; in MOPS buffer, 21.0 (100mM). The medium pH was adjusted to pH 6.8 with titration of NaOH 5 M solution and then sterilized. Reactivated cells were then inoculated in the respective mediums used in the CA production assays.
In the next phase, the semi synthetic medium GSPA was used.10 GSPA medium composition was (g.L-1 in distilled water): glycerol, 15.0; sucrose, 20.0; proline, 2.5; glutamic acid 1.5; arginine, 10.0; NaCL, 5.0; K2HPO4, 2.0; CaCl2, 0.4; MnCl2.4H2O, 1.0; FeCl3.6H2O, 0.1; ZnCl2, 0.05; MgSO4.7H2O, 1.0; pH 7.0. This specific medium was used due to good results in our laboratory investigating production of CA with mutant strains in a previous study.12
Culture conditions
Batch cultivations for the S. clavuligerus CA production were done in a shaker G-25 (New Brunswick Scientific Co). The assays were done in three steps, strain reactivation, microorganism growth and CA production. In the reactivation step, 3.5mL of the S. clavuligerus vegetative cell suspension stock was inoculated in 500mL Erlenmeyers containing 50mL of reactivation medium, and incubated for 24h at 28°C, 250rpm.
In the growth step, 5mL of the reactivated S. clavuligerus suspension was transferred to 500mL Erlenmeyers containing 45mL of culture mediums, each one with the same composition relative to the main CA production mediums. The growth step was carried over 24h at 28°C, 250 pm. Finally, in the production step, the whole growth culture volumes were transferred into Erlenmeyers containing the production mediums, beginning the main CA production process. CA production cultures were carried over 120h and the samples from the cultures were collected every 12h.
Biomass and clavulanic acid determination
Cellular biomass was analyzed based on the dry weight method. First, the cells were collected by centrifugation of the samples, washing the cells twice with double distilled water. The samples were then incubated at 65°C until reaching the measured constant weight. CA concentration was determined by HPLC using the Foulstone & Reading16 method, in a C-18µ-Bondapack column. The mobile phase was a methanol/phosphate buffer mix, at 2.5mL/min-1 flow rate. Elution temperature was kept at 28°C and was monitored at 311 nm wavelength. A reference calibration curve was previously done using an amoxicillin/potassium clavulanate mix (the content of Clavulin 250mg - Smith Kline-Beecham do Brasil Ltda).
Statistical analysis
All the analyses and experiments were performed in triplicate. Data are shown as means ±SEM. Differences between the WT and Mutant groups were compared by the unpaired t-test. To compare changes in each variable over time one-way ANOVA was performed followed by the Tukey and Kramer post-hoc test. The differences were considered significant at P<0.05. Data were analyzed using GraphPad InStat 3.00 software (GraphPad Software, San Diego, CA, USA).
CA is an industrially important secondary metabolite due to its inhibitory action on b-lactamases. There are many examples of strain improvement strategies applied to S. clavuligerus in order to reach high levels of CA production with genetic engineering and random mutagenesis.9,17
In this study, we used random mutagenesis and our results showed that CA production increased 28% within the mutant strain when compared to the wild-type strain. The level of CA reached a maximum after 72h of cultivation in mutant and wild-type strains. The mutant strain obtained 812mg L-1 concentration in 72 h of fermentation, while the wild-type obtained 639.7.1mg L-1 at the same time (Figure 1). This concentration of CA in the mutant strain was similar at our previous study,12 in which only the chemical mutagenesis was used.
The significant increase in biomass in the mutant strain compared to wild type (Figure 2), suggests that the mutations occurred in the genes which are involved in the primary metabolites responsible for growth. It might be interpreted that the overproduction of CA, following the mutations, is a result of overgrowth. We also suggest that mutations in the genes involved in the metabolic pathway of CA production may have eliminated the competing pathways of structurally similar molecules, leading to a more efficient CA production.
According to Medema et al.,18 random mutagenesis can cause gene transcript changes in both primary and secondary metabolism. Some random mutagenesis were strikingly similar to those rationally engineered by two strategies that have recently been employed to increase CA production in S. clavuligerus: redirection of carbon fluxes towards the key CA precursor glyceraldehyde-3-phosphate (G3P), and upregulation of pathway-specific activators.18 In this study, the highest productivity of the two strains occurred in 72h of cultivation. The productivity of the wild-type and the mutant strains were 8.88 and 11.28mg L-1 h-1 respectively (Table 1).
These results show that although laborious and empirical, the strategy of random mutation and screening can be a good method for improvement in CA production. The improvement of microbial strains plays an important role in reducing production costs during industrial fermentation. Korbekandi et al.9 reported that the concentration of CA was increased 1.8 times after UV mutagenesis. In another study, Said et al.19 show that relatively safe mutagens like UV represent a very convenient system to enhance strains through random mutation. On the other hand, only a small number of researchers have utilized UV irradiation to overproduce CA. According to Baltz,20 chemical mutagenesis is a very successful method to improve secondary metabolites in Streptomyces. It is important to emphasize that the GSPA culture medium was selected because this medium has high concentration of arginine. Indeed the primary metabolic precursors of CA are D-glyceraldehyde-3-phosphate (G3P)21 and L-arginine.22 Li & Townsend11 have already shown that the addition of arginine to the cultured mutant strain further improved CA production giving a greater than two-fold increase over the wild-type. In addition, the authors show that this amino acid is fundamental for CA biosynthesis and your lack is a limiting factor.
This study showed that random mutagenesis like chemical and UV irradiation are methods safe and can be used for improvement AC production as well as other biosynthesis metabolites, altering genes and consequently levels of these substances. More guidance acts are necessary to inappropriate use and excessive treatment of antibiotics, thereby avoiding several human deaths. Additionally, a better understanding of the mechanisms involved in the production of CA as well as studies aimed at increasing production it is necessary.
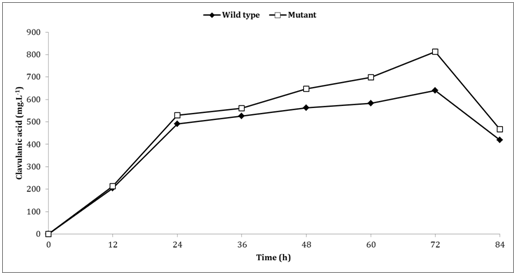
Figure 1 Comparison of clavulanic acid production between the mutant and wild-type strains cultivated in semi-synthetic mediums GSPA..

Figure 2Comparison of biomass between the mutant and wild-type strains cultivated in semi-synthetic mediums GSPA. Mean values ± SEM. Asterisks indicate significant differences between wild-type and mutant strains (P < 0.05).
Strain |
Maximum CA concentration (mg L-1) |
Production time (h) |
Productivity (mg L-1 h-1) |
Wild type |
639.72 |
72 |
8.88 |
Mutant |
812.71 |
72 |
11.28 |
Table 1 Maximum clavulanic acid concentration and productivity of wild-type and mutant strains
Our findings clearly demonstrate that random mutations can lead to the improvement in CA production in S. clavuligerus. The highest levels of CA production obtained within the mutant strain reached 28% more than the levels of the wild-type strain. However, adding another mutation in the same mutant strain, in this case, UV light does not improve the production. The semi synthetic medium GSPA promoted an excellent biomass growth and CA production with both strains. This work reinforces the importance of using random mutagenesis methods in S. clavuligerus for biotechnological research of productivity improvement.
The authors declare there is no conflicts of interest.

©2018 Vasconcelos, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.