Journal of
eISSN: 2469 - 2786


Cattle are one of the farm animals of huge economic significance as the main source for meat and milk production in Egypt but their productivity is thought to be greatly reduced by blood protozoan parasitic diseases caused by tick-borne blood parasite such as babesiosis and theileriosis. Babesiosis and theileriosis are one of the most important and serious pathogenic protozoan diseases infect these animals in Egypt with highly prevalent and caused by Babesia and Theileria species.
The most practical and widely used method to control such disease is the chemical control of ticks with acaricides and treatment of infected animal but both way of control are not always fully effective and hence vaccination is the most sustainable option. Many epidemiological factors are responsible for causing parasitic infections in cattle. So, proper monitoring, diagnosis, immunization and control of parasitic infections in cattle in Egypt are required for sustainable growth and proper development of cattle management program.
Therefore, knowledge of prevalence of blood protozoan parasites and current species will help to minimize the economic losses in cattle sector. So this short review might have been spotted the light on the current prevalence of such infections in cattle in Egypt with special attention to diagnostic tools and treatment used for this purpose
Keywords: cattle, babesiosis, theileriosis, prevalence, diagnosis, east coast fever, indirect fluorescent antibody test, enzyme linked immunosorbent assays, polymerase chain reaction
T, theilaria; ECF, east coast fever; IFAT, indirect fluorescent antibody test; ELISA, enzyme linked immunosorbent assays; PCR, polymerase chain reaction
Egypt, cattle are very important economically because they are the main sources of animal protein and income. Their by-products such as hoof, bones, blood, hides and skin are also variously used.1 Beef is the third most widely consumed meat in the world, accounting for about 25% of meat production worldwide, after pork and poultry at 38% and 30% respectively.2 Furthermore, beef is an excellent source of complete protein, minerals such as zinc, selenium, phosphorus and iron and the B vitamins. Nowadays Haemoparasitic infections are major public health, veterinary and socio-economic problems in Africa, where they impose a burden on the healthcare infrastructure of both animals and animal handlers in endemic areas.
Babesiosis and theileriosis are apicomplexan–hemoprotozoan parasites transmitted by Ixodidae ticks3 and are observed to be devastating parasites affecting the production of livestock, mainly cattle. These infections are of worldwide significance and are characterized by anemia, icterus, hemoglobinuria, and death, and as a result, they have a high economic impact in several parts of the world, including tropical and temperate countries.4
Moreover, they impair cattle improvement programs due to their imposing significant economic burden on meat and milk production beside to costs of treatment and tick control. They are currently considered as the most important endemic parasitic diseases affecting cattle in Egypt.5
Bovine babesiosis is a febrile, haemolytic disease caused by intraerythrocytic protozoan of the genus Babesia, including several species namely Babesia bigemina, B. divergens, B. bovis, and B. major. Additionally, B. bovis and B. bigemina are the main species affecting cattle widely distributed throughout Egypt6 and other tropical and subtropical countries which are responsible for high mortality rates up to 50% in susceptible herds7 and it's known to be transmitted in this country by Rhipicephalus microplus where as Babesia bigemina is known to be transmitted by both Rhipicephalus. Microplus and Rhipicephalus. Annulatus.
Babesia bovis is generally more pathogenic5 Theilerioses, on the other hand is a group of tick borne disease caused by Theilaria (T) species; the two most pathogenic and economically important are T. parva and T. annulta.8 So globally the most common cause of bovine theileriosis is T. annulata and T. parva.9
parva occurs in 14 countries10 in sub-Saharan Africa causing East Coast fever (ECF) which is characterized by enlargement of superficial lymph nodes and a sustainable fever11 and still ranks first among the tick-borne diseases of cattle in sub-Saharan Africa12 whilst T. annulta occurs in southern Europe as well as North Africa and Asia causing Tropical Theileriosis.8
Babesiosis
The disease also called piroplasmosis, tick fever, Red water fever or Taxes fever.13
Etiology
The genus Babesia belongs to the phylum Apicomplexa, class Sporozoasida, order Eucoccidiorida, suborder Piroplasmorina and family Babesiidae14 used the 18s rRNA gene for phylogenetic analysis.
Morphology
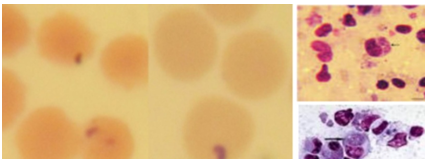
Babesia bovis is a small parasite, usually centrally located in the erythrocyte. It measures approximately 1-1.5μm long and 0.5-1.0μm wide, and is often found as pairs that are at an obtuse angle to each other. While, Babesia bigemina is a much longer parasite, and is often found as pairs at an acute angle to each other. Babesia bigemina is typically pear-shaped, but many diverse single forms are found. It is 3-3.5μm long and 1-1.5μm wide, and paired forms often have two discrete red-staining dots in each parasite (B. bovis and B. divergens always have only one) (Figure 1 & 2).15

Pathogenesis
Babesia species produces acute disease by two principle mechanism; hemolysis and circulatory disturbance.16 During the tick bite, sporozoites are injected into the host and directly infect red blood cells. It invades erythrocyte and cause intravascular and extra vascular hemolysis.17The rapidly dividing parasites in the red cells produce rapid destruction of the erythrocytes with accompanying haemoglobinaemia, haemoglobinuria and fever. This may be so acute as to cause death within a few days, during which the packed cell volume falls below 20% which will lead to anemia.18
Clinical picture of disease
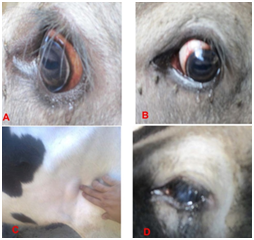
Affected animals suffered from marked rise in body temperature, loss of appetite, cessation of rumination, labored breathing, emaciation, progressive hemolytic anemia, various degrees of jaundice (Icterus) from paleness in mild cases to severe yellow discoloration of conjunctival and vaginal mucous membranes in more progressive cases haemoglobinuria, accelerated heart and respiratory rates, ocular problems and drop in milk production. The fever during infections in some cases cause abortion to pregnant cattle.19 Coffee colored urine is the characteristics clinical feature of Babesiosis. Babesiaspecies cause both acute and persistent subclinical disease in cattle.20
Pathological features
Lesions include an enlarged soft and pulpy spleen, a swollen liver, a gall bladder distended with thick granular bile, congested dark-colored kidneys and generalized anaemia and jaundice. Other organs may show congestion or petechial haemorrhages and occasionally there will be pulmonary oedema. The grey matter surface of the brain can appear pink. Acute cases will show haemoglobinuria, but this may be absent in subacute or chronic cases. Clinical pathology emphasis on a haemolytic anaemia, which is characteristically macrocytic and hypochromic. Hematological, biochemical and histopathological changes are described by de DeVos & Potgieter (Figure 3).21
Etiology
Theileria species, tick transmitted, intracellular protozoan parasites infecting leukocytes and erythrocytes of wild and domestic large and small ruminants. In cattle: T. parkas, T. annulata, T. mutans, T. velifera, T. tarurotragi and T. buffeli.22 T. annulata and T. parva are considered to be the most pathogenic species of Theileria affecting cattle.23
Morphology
The piroplasm forms in the red blood cells more commonly occur as round, oval, or ring shaped (0.5-1.5µm) forms. Rod, comma (1.6µm) shapes and anaplasma - like organisms may also be found, the latter measuring 0.5µm. Macroschizonts and microschizonts are found in the lymphocytes of the spleen and lymph nodes, that are known as schizonts (Koch's blue bodies), being circular or irregularly shaped structures about 8µm in diameter, but vary from 2 to 12µm or more. Two forms of schizonts are recognized, those which contain large chromatin granules (0.4-2µm in diameter with a mean of 1.0µm) that are referred to as macroschizonts and produce macromerozoites (2-2.5µm diameter). The other forms contain smaller chromatin granules (0.3-0.8µm in diameter with mean of 0.5µm) and are referred to as microschizonts that produce micromerozoites (Figure 4 & 5).24
Pathogenesis
Theileria spp. can be grouped into schizont “transforming” and “non-transforming” species. Nontransforming Theileria is regarded as being but still able to cause disease as a result of anaemia induced by the piroplasm stage.25 Pathogenesis of various forms of Theileriosis is dependent on the production of schizonts in lymphocytes and piroplasms in erythrocytes.26 Moreover, the severity of infection depends upon virulence of the causative strain, the quantum of infection, the susceptibility status, age and health of the host.27 The parasite replicates in both lymphocytes and erythrocytes causing severe lymphocytopenia, anaemia and jaundice.
Clinical picture of the disease
Tropical Theileriosis of cattle characterized by lymphproliferative disorders associated with lymph nodes enlargement in its early phases. The first lymph nodes involved are the pre scapular lymph node as a result of the predilection sites of the vector ticks and by lymph destructive disorders associated with fever, pronounced leukopenia, diarrhea, lateral recumbency and marked anemia in its late phases, weight loss, weakness, anorexia, conjunctival petechia and cough are the most common symptoms.28
On the other hand, the clinical signs of East Cost Fever (ECF) characterized, unlike Tropical Theileriosis, by the absence or limited intensity of anemia and high frequency of respiratory sign. Other symptoms include; diarrhea, corneal opacity leading to blindness and subcutaneous edema.29 In susceptible cattle, ECF is characterized by enlargement of superficial lymph nodes starting with the parotid lymph node, a sustainable fever and diverse clinical signs associated with invasion of non-lymphoid tissues with parasitized lymphoblast. ECF causes high mortality with death occurring approximately three weeks after infection, mainly as a result of severe pulmonary oedema.8
Pathological features
The most prominent pathological features of tropical theileriosis are enlargement and swelling of the superficial and internal lymph glands and spleen, and lesions are generally in the form of ecchymotic and petechial haemorrhages of most of the internal organs, kidney infarcts liver degeneration, lung oedema, serous and mucous membranes and ulcers on the abomasal mucosa which may extend to intestines.30
Status of babesiosis and theileriosis in different localities in Egypt. Similar to other countries, there are a considerable number of economically important livestock diseases occurring in Egypt. Among others, tick borne haemoparasitic diseases are of the major constraints to the livestock industry of the country. The variable occurrence rates of babesiosis and theileriosis among cattlein Egypt have been reported by numerous studies such as:35 conducted a study to assess the prevalence of babesiosis and theileriosis at Behera province, Assuit by using thin blood smears, stained with Giemsa stain under microscopy. It was found that 43.3% of cattle were positive. Then the incidences of Babesia spp. was 19.33 %, while the incidence of Theileria spp. was 23 %.36
Reported that Babesia spp. were detected in 11.31%, 8.15%, and 23% of cattle in Gharbia, Menofia and Port Said Governorates, Egypt. Furthermore, in Damietta governorate,37 by using blood smear examination found that the prevalence of Babesia spp. in cattle was 46.9% and the same result by28 who detect 38.5%of tested cattle were found to be positive for Babesia spp. in Menofia Governorate.
Additionally, Abdel Aziz KB et al.18 through using microscopic examination, cELISA and nPCR observed that the overall prevalence rate of babesiosis was 12% by microscopic examination. 44% represented a positive result to specific antibodies of babesiosis, with 21.6% positive for Babesia bigemina, 10.8% positive for Babesia Bovis. However, in nPCR, 39.5% samples were positive for babesiosis in which 24.7% were positive for Babesia bigemina and 14.8% were positive for Babesia bovis (Figure 6 & 7).
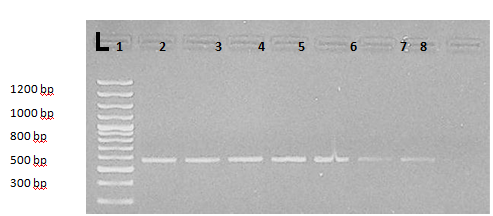
El Moghazy et al.17 reported that, the infection rate with Babesiosis was 22.47%, while the infection rate of Theileriosis was 14.61% in Qalubia Governorateusing blood film examination. Whereas PCR assay, revealed that the infection rate of babesiosis was 75%. Additionally, the results specified that the young age was more susceptible to tick borne parasites infections as compared to adult.
Mahmoud et al.38 by using blood film examination their study revealed that the infection rate of Babesia was 13.8 % in cattle. However, the infection rate of Babesia bigemina and Babesia bovis was 32.8% and 21.3 % respectively, by using the cELISA technique.
On the other hand, molecular biological identification of Babesia, Theileria by Maged et al. (2015) in cattle in the Dakahlia Governorate of the Delta region of Egypt using PCR assays, gene sequence analysis, and a novel DNA microarray showed that the rate of animals infected with Babesia bovis and Babesia bigemina was 7.3% and 1.2%, respectively.
Moreover, for Theileria spp., different researchers have reported the prevalence of bovine theileriosis from different area of Egypt like, Nayel et al.28 presented that the overall prevalence was 16.05 % in Menofiya Governor Rate and39 in Qena province (11.1 %) and40 in Gharbia province (11.31 %). Furthermore,41 who revealed the prevalence, rates of Theileria spp. was 65% in cattle, and42 in El-Behera province, who found that the prevalence of Theileria annulata was 65.4 % using stained blood films.
Ibrahim et al.6 found that the prevalence of Babesia spp. and Theileria spp. were 26% and 22% respectively as detected by PCR using a common primer while by microscopic examination the results were 22% and 13% for Babesiaspp. and Theileriaspp. Respectively in Giza Governorate. Of which 17%, 9% and 13% were positive for Babesiabigemina, Babesia bovis and Theileria annulata specific PCR. Using of ELISA revealed higher babesiosis and theileriosis infection percentage than that of PCR, as 39% and 28% respectively.
The seroprevalence of protozoan infections in cattle in Qena and Sohag governorate, Upper Egypt was determined with enzyme-linked immunosorbent assays using species-specific diagnostic antigens as reported by Ragab et al. (2017) in his study, they found that 33.2% and 42.2% of examined cattle were found to be positive for specific antibodies against Babesia bovis, Babesia bigemina, respectively.
On other hand,43 revealed that 25.8% of the tested cattle were positive for Theileria infection by blood film. Additionally, the incidence of Theileria infection in the different localities was 17.5%, 20% and 40% in the Assiut, Sohag and El-Wadi El-Gadid governorate, respectively. Cattle management, climatic condition, immune status, tick distribution, breeds, and the sampling condition might explain the variation in prevalence rates between previous studies in Egypt.
Control of babesiosis and theileriosis is mainly based on the chemotherapy and tick eradication in Egypt. For babesiosis, Imidocarb dipropionate salt (Imizol) is effective. Diminazene aceturate (Berenil) is widely used. Another supportive therapy is important in chronic cases and convalescent animals such as vitamins, electrolytes and hematinics drugs.
Treatment of theileriosis infection in cattle depending on the chronicity of the disease, this achieved by using three drugs, buparvaquone (Butalex, Schering-Plough), oxytetracycline and Berinil (Dimenazine citrate). The results revealed that Butalex was effective in treating cases suffering from acute theileriosis. However, oxytetracycline and Berinil were effective in treating cases with chronic theileriosis. Early treatment was highly efficient in the elimination of protozoan parasites from blood within 3-4 days post-treatment with a clinical improvement.45‒50
The occurrence of babesiosis and theileriosis among cattle in Egypt, suggests the need for appropriately designed prevalence studies to cover most of the regions in this country with proper diagnostic tools combined with the understanding of the immune complex response will facilitate the development of improved methods for monitoring, control, immunization and eradication of such disease. Therefore based on the above conclusions the following recommendations can be proposed:
None.
The author declares no conflict of interest.

© . This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.