Journal of
eISSN: 2473-0831


Research Article Volume 6 Issue 3
Correspondence: M
Received: October 27, 2017 | Published: November 7, 2017
Citation: Márcio RMS, Elisabete PS, Rita CSAB, Sheila G (2017) Validation of a New RP-HPLC Method for Determination of Hydrocortisone Acetate Complexed with HP?CD in a Oral Liquid Pharmaceutical Preparation. J Anal Pharm Res 6(3): 00180. DOI: 10.15406/japlr.2017.06.00180
A simple reversed-phase high-performance liquid chromatographic method (RP-HPLC) for the simultaneous determination of hydrocortisone acetate (HA) complexed with 2-hydroxypropyl-β-cyclodextrin (HPβCD), its degradation products and preservative in an oral liquid pharmaceutical preparation has been validated. The compounds were separated on a 150mm x 4.6mm C 18 column packed with 5μm particles and a 1mm x 4mm pre-column with heating at 30 °C. The mobile phase optimized was a mixture of methanol, acetonitrile and water 35:25:40 (v/v/v), pumped at a flow rate of 1.0mL min-1. The validation data showed that the proposed method is simple, reliable, fast and has good robustness, specificity, linearity, precision and accuracy.
Keywords: hydrocortisone acetate, RP-HPLC, validation and HPβCD
Hydrocortisone acetate (HA) is a synthetic corticosteroid and is usually employed in the treatment of classic congenital adrenal hyperplasia (CAH), which is a disorder of the adrenal cortex characterized by cortisol deficiency, with or without aldosterone deficiency, and androgen excess.1 Because five enzymatic steps are involved in cortisol biosynthesis, there are five distinct CAH syndromes.2 Approximately 95 percent of cases are due to 21-hydroxylase deficiency.3 The clinical features are; virilization, rapid somatic growth and accelerated skeletal maturation.4
The HA is the best therapeutic choice in the CAH treatment, especially in children, because to be the hormone more similar to endogenous cortisol.5 The HA show poorly water solubility and instability due to suffer hydrolysis.6 One method to overcome this problem is the use of cyclodextrins (CDs), which have been extensively studied.7
CDs are macrocyclic torus-shaped molecules formed by D - (+) - glucopyranose units. CDs size and shape are correlated to the type and number of (1,4) linkages between those units and contain a somewhat lipophilic central cavity and a hydrophilic outer surface. CDs are capable of forming inclusion complexes with various types of lipophilic drugs with improve solubility, stability or other physicochemical properties.8 Complexation of molecules to CDs occurs through a non-covalent interaction between the molecule and the CDs cavity.9
The 2-hydroxypropyl-β-cyclodextrin (HPβCD) is a chemical modified CD, where the group 2-hydroxypropyl replaces hydrogen atoms of the free groups.10 Nowadays, HPβCD is the most versatile excipient among the cyclic oligosaccharides; it can be used in oral, rectal, dermal, ocular, and parenteral formulations. There are several marketed pharmaceutical products containing HPβCD with various active ingredients. The formulations are mostly solutions (infusion, injection, and eye drops) utilizing the solubilizing and stabilizing effect of HPβCD.11
HA is currently available only in injection, eye drops, cream and ointment at Brazilian market. Some works make reference to the simultaneous determination of HA from others compounds in formulations by RP-HPLC12-15 and only one mentioned the simultaneous determination of HA and preservatives in the presence of its degradation products.16
An official method for simultaneous determination of HA complexed with HPβCD and others compounds (degradation products and potassium sorbate in oral liquid formulation has not been described. In this work, we have prepared an oral liquid pharmaceutical of HA complexed with HPβCD according phases solubility diagram of Higuchi & Connors. The aim of the study was to develop and validate an analytical method by RP-HPLC for simultaneous determination of HA assay complexed with HPβCD its degradation products and potassium sorbate as preservative in an oral liquid pharmaceutical preparation.
Reagents
HA active substance (Figure 1) and potassium sorbate (preservative) were purchased from Galena - Brazil. HA reference substance was obtained from Sigma - Aldrich, Brazil. The HPβCD was kindly donated by Cargill-USA. Methanol and acetonitrile HPLC grade were obtained from Tedia. Methanol was purchased from Vetec-Brazil. The water used was ultra-pure grade.
Apparatus
The HPLC system consisted of a Shimadzu LC-10 ADVP pump, a SPD-M 10AVP photodiode array detector, a SCL-10 AVP system controller, SIL-10 ADVP auto injection and a degasser module. The stirrer with heating equipment was used for preparation of HA/HPβCD inclusion complex. A dry air oven was used for degradation studies of HA.
Preparation of HA/HPβCD inclusion complex
The inclusion complex of HA and was prepared according to phase solubility diagram method of Higuchi & Connors. The phase solubility profiles obtained for the complex formation between HA and HPβCD system, showed that the aqueous solubility of the drug increase linearly as a function of HPβCD concentration. The shape of solubility diagram represents linear host-guess correlation (AL-type) with slope less than 1 indicating the formation of 1:1 (mol/mol) complex with HPβCD.17 In addition, in work performed by Jelena et al.18 assumed that a 1:1 complex was initially formed between HA and HPβCD.18
Preliminary experiments were carried out in order to determine the equilibrium. There were exactly weighed about 50.0mg of HA on conical flask of 125mL and added a volume of a HPβCD solution 150mM in water until a concentration of 135mM. The total volume inside the flask was 25,0mL. The flask contained a concentration relationship of 5mM and 135mM from HA and HPβCD, respectively. The conical flask containing the sample had been covered, protected of the light with aluminum foil and placed for one hour in water bath (37ºC), under constant magnetic stirring. After reached the equilibrium, the samples were filtered through a filter paper (Whatman® n°41). The sample was freeze-dried.
Development of oral liquid pharmaceutical preparation
For the preparation of oral liquid pharmaceutical HA/HPβCD were used the preservative potassium sorbate (0,2%) and simple syrup (80% from sucrose). The preservative (potassium sorbate) was dissolved in water with heating at 65 °C and constant stirring. After cooling at a room temperature, the solution was filtered and added in the simple syrup. The HA/HPβCD freeze-dried previously was solubilized in water through of ultrasound by five minutes. After, the resulting solution was incorporated into solution containing the simple syrup and preservative. The pH of the preparation was checked through a potentiometer. The pH was around 5,0.
Chromatographic conditions
The chromatographic column used was a C18 150mm x 4mm i.d with 5μm particles coupled to a guard column 10mm x 4mm i.d 5μm. The mobile phase was selected as methanol, acetonitrile and water in ratio 35:25:40 v/v/v, respectively. The mobile phase was filtered on membrane filter 0,45μm (millipore®). The flow rate was 1,0mL min-1 and sample injection volume was 10μL. The detection was achieved with photodiode array detection at 238nm. The temperature in the column oven was 30 °C.
Sample preparation
Six units of preparation were separately weighed and transferred to a 10mL volumetric flask and after diluted with 10mL of methanol:water (50:50 v/v) to generate a final concentration of 240μg mL-1. The samples were filtered using a 0,45μm nylon filter before the injection.
Standard preparation
HA reference substance was accurately weighed (50,0mg) and dissolved in a 50mL volumetric flask of ethanol, this is stock solution. The stock solution was diluted with a mixture of methanol:water (50:50v/v) to give a range concentration of 240 to 360μg mL-1. The samples were filtered using a 0,45μm nylon filter before the injection.
Degradation of HA
For analyze the interference with the analytical method were investigated potential HA degradation products through forced decomposition tests. Samples of HA active substance were submitted a forced stress condition, by a dry air oven at 40 °C during two days. This test was performed before the use of HA in the formation of the HA/HPβCD inclusion complex.
Method validation
Establishing documented evidence that provides a high degree of assurance that a specific method, and the ancillary instruments included in the method, will consistently yield results that accurately reflect the quality characteristics of the product tested.19 The developed HPLC method for the analysis of HA complexed with HPβCD in an oral liquid formulation was validated according to ICH Q2(R1) and RDC 166 (ANVISA 2017).20,21
Robustness
Robustness is defined as a measure of the method´s capability to remain unaffected by small, but deliberated variations in the method parameters.21 To evaluate the robustness of the method slightly changes in the chromatographic conditions were determined, such as mobile phase in different concentrations of components; percentage of methanol of 28 and 35% and acetonitrile of 32% and 35%, flow rate of 1,0 and 1,2mL min-1, guard column and column temperature at 25 and 30°C and wavelength at 238nm and 242nm.
Specificity
The specificity is the ability to assess unequivocally the analyte of interest in the presence of those components which may be expected to be present in the sample matrix. Specificity/selectivity is the most crucial parameter of any analytical method used for stability and assay determination.21 The specificity of the method was established through of the analysis of formulation with excipients and without the HA/HPβCD inclusion complex, the preparation with excipients, HA/HPβCD inclusion complex, degradation products of HA previously obtained by forced stress condition of HA active substance and with only the HPβCD. In order to verify the interference of the excipients in the analytical method.
Linearity
The linearity of analytical method is its ability to elicit test results that are directly proportional to the concentration of analytes in sample within a given range. The range of analytical method is the interval between the upper and lower levels of analytes that have been demonstrated to be determined within a suitable level of precision, accuracy and linearity.22 Test solutions were prepared from stock solution of HA standard in ethanol (1000μg mL-1), aliquots were transferred to volumetric flasks with mixture of methanol:water (50:50 v/v) to obtain five final concentrations (240, 270, 300, 330 and 360μg mL-1). The samples were filtered using a 0,45μm nylon filter before the injection. Each one was prepared in duplicate and was injected in duplicate, this procedure was made in triplicate. The slope, y-intercept and correlation coefficient were calculated.
Precision
The precision of the assay method was evaluated by six independent sample solutions of HA in the preparation at the same concentration (240μg mL-1), during the same day. The samples were filtered using a 0,45μm nylon filter before the injection. Each one was prepared in triplicate and was injected twice. The relative standard deviation (RSD%) of measures was calculated. The RSD less than 2% is sufficiently precise.20
Accuracy
The accuracy of an analytical method should be obtained by the degree of agreement between the individual results of the method under study in relation to a value accepted as true.21 The accuracy of the method was determined in triplicate at three concentration levels, i.e., 240, 300 and 360μg mL-1. The samples were filtered using a 0,45μm nylon filter before the injection.
Degradation of HA
After stress conditions 40 °C during two days were found two potentials degradation products of HA active substance. The first with a run time around 3 minutes and the second around 4 minutes.
Selection of chromatographic conditions (Robustness)
Changes in some parameters of the analytical method were tested. Mobile phase with different proportions of organic solvents (methanol and acetonitrile), flow rate, column temperature and detection were checked to improve the separation and peaks resolution and run time. The ideal condition of analysis was the following; mobile phase (methanol, acetonitrile and water 35:25:40 v/v/v), flow rate 1.0mL min-1, guard column and column temperature 30 °C and detection at 238nm. The retention time of potassium sorbate was 2.976 minutes, for HA degradation products were 3.915 and 5.237 minutes and for HA was 5.899 minutes is showed in Figure 2. The total run time of proposed method was around 6 minutes, this time is less than that method developed by Hájková et al.16 which was around 13 minutes, as showed in Figure 3. In that work, the conditions of the analysis were performed on a 5 mm SUPELCO C18 125 x 4 mm ID column with pre-column 20 x 4 mm ID, 5 mm. The optimal mobile phase for separation was a mixture of methanol, acetonitrile and water in ratio 15:27:58, v/v/v. The injection volume 10 ml, at a flow rate 0.8 ml min-1, the detection wavelength 238 nm.16
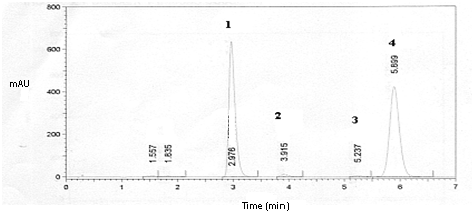
Figure 2 Chromatogram of HA complexed with HPβCD in oral liquid pharmaceutical preparation where; 1 potassium sorbate, 2 and 3 degradation products and 4 HA.
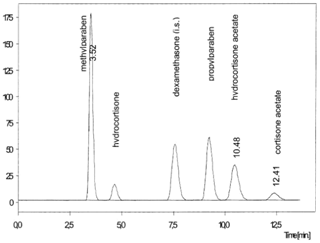
Figure 3 Chromatogram of method developed by Hájková et al.16 HA run time was around 10 minutes.
Specificity
The specificity of method was evaluated through the presence of excipients, HPβCD and preservative (potassium sorbate) in the oral liquid pharmaceutical preparation, without the HA/HPβCD inclusion complex. The run time from the preservative was around 2 minutes. Figure 4 showed that excipients, HPβCD and preservative not interfered on the analysis. The chromatogram of preparation containing excipients, the HA/HPβCD inclusion complex and degradation products demonstrated that any peak from preservative and degradation products interfered with HA peak (Figure 2). These results indicated that this method has a high degree of specificity for determination of HA complexed with HPβCD in the preparation.
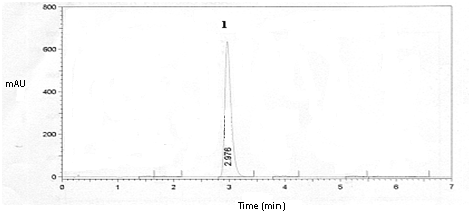
Figure 4 Chromatogram of excipients, HPβCD and preservative in oral liquid pharmaceutical preparation, without the HA/HPβCD inclusion complex. where; 1 is the peak of preservative (potassium sorbate).
Linearity
Linearity was established by least squares linear regression analysis of calibration curves. The regression analysis for HA was found by plotting peak area (y) versus the concentration of HA (x) analyzed from 240 to 360μg mL-1.The average of representative linear equation was: y=22922x - 435847 and the correlation coefficient r= 0.996 (Figure 5). The drug response with respect to peak area was linear over the concentration range 240-360μg mL-1 of HA
Precision
Six solution samples of HA in the preparation were prepared at (240μg mL-1) concentration and each one was determined in triplicate and injected twice. The analyses were performed at the same day. The precision results RSD% of the assay was 0.76% (Table 1). This value demonstrated that the method is reproducible. The RSD% found on this method was better than the method developed by Hájková et al.16 that was RSD% 1,46.
|
Weight of Preparation (g) (n = 6) Mean |
Assay (%) (n = 6) |
|
2.0440 |
100.4 ± 0.76 |
Table 1 Intra-day precision for determination of HA assay of preparation.Intra-day precision for determination of HA assay of preparation
Accuracy
The experimental results at each level of HA concentration (240, 300 and 360μg mL-1) were determined. The results calculated for accuracy ranged from 99.1% to 102.6% and RSD% was 1,01 (Table 2) indicated that this method is reliable and these values show the good accuracy of the proposed method. The RSD% found was better than the method developed by Hájková et al.16 that was RSD% 1,98.
|
Teorical Concentration (μg mL-1) |
Recovery Assay (%) |
RSD% |
|
239.1 |
101.8 |
1.01 |
|
240.6 |
102.6 |
|
|
242.2 |
100.7 |
|
|
308.4 |
100.8 |
|
|
306.2 |
102.2 |
|
|
310.8 |
101.1 |
|
|
361.6 |
101.1 |
|
|
364.6 |
100.9 |
|
|
363.4 |
99.1 |
Table 2 Accuracy of the analysis of HA in preparation
In this present work was described a new, simple, reliable and fast RP-HPLC method for the simultaneous determination of assay HA complexed with HPβCD, its degradation products and potassium sorbate in the oral liquid pharmaceutical preparation, with a short run time of around 6 min. There was no interference from any excipients in the determination of HA which indicates the method is specific. Furthermore, validation parameters were performed and suitable robustness, specificity, linearity, precision and accuracy were found. The method validation parameters lie within its acceptance criteria as per ICH Q2(R1) guideline and RDC 166 (ANVISA 2017). Hence, it can be concluded that the developed and validated RP-HPLC method is useful for the quantification of HA in oral liquid pharmaceutical preparation.
Any financial interest or any conflict of interest exists in the present work.
None.

©2017 Márcio, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.