Journal of
eISSN: 2473-0831


Mini Review Volume 2 Issue 1
Correspondence: Paul McCarron, School of Pharmacy and Pharmaceutical Sciences, SAAD Centre for Pharmacy and Diabetes Ulster University, Cromore Road, UK, Tel 7792156770
Received: September 23, 2015 | Published: January 13, 2016
Citation: AbdelKader DH, Osman MA, Elgizawy SA, Faheem AM McCarron PA (2016) The Role of Insulin in Wound Healing Process: Mechanism of Action and Pharmaceutical Applications. J Anal Pharm Res 2(1): 00007. DOI: 10.15406/japlr.2016.02.00007
Skin is a complex tissue, which comprises multiple layers. An injury to this stratified structure is considered to be the beginning of a sequence of events designed to restore skin integrity. Depending on the diameter and severity of a wound, deleterious physiological and metabolic changes can occur, leading to impaired wound healing and increased morbidity and mortality. While wound dressings provide some form of protection and remedy, the main challenge is to restore local metabolic pathways to normality, especially in the comprised patient suffering from chronic illness, such as diabetes. The implications of this disease in particular have prompted investigations into topical insulin as a potential and promising therapeutic intervention. This mini-review describes the possible mechanisms of insulin that are responsible for stimulating the cellular and molecular pathways, thereby enhancing the wound healing process. Examples of systemic and topical insulin applications are mentioned, together with an evaluation of the critical role played by insulin in tissue regeneration.
Keywords: insulin, wound healing, keratinocyte migration, akt pathway, topical delivery
PI3K-Akt, phosphatidylinositol-3-kinase and protein kinase B; Rac1,ras-related C3 botulinum toxin substrate 1; LN332, laminin 332; EGF, epidermal growth factor; EGF-R, epidermal growth factor receptor; IGF-1&IGF-II, insulin-like growth factor 1&2; Gtpase, guanosine 5'-triphosphate hydrolase enzyme; IRS-1 IRS-2, insulin receptor substrate 1&2; ERK, extracellular receptor kinase; VEGF,vasoactive endothelial growth factor; SOS, the son of sevenless; Enos: endothelial nitric oxide synthase; SDF-1α, stromal-derived-factor-1; MIP-2, macrophage inflammation protein 2.
The economic cost of wound management has been estimated to be measured in billions of dollars. Current financial projections indicate that the cost of managing wounds accounts for almost 4% of total health care expenditure, and that this spiralling burden shows no sign of slowing down. Recalcitrant, non-healing wounds constitute a major problem that plagues those patients with chronic illness, such as diabetes. Despite systemic insulin treatment and a carefully regulated life style, approximately 15% of all diabetic patients will have some form of non-healing wound and be particularly susceptible to amputation of the lower extremities.1,2
Wound healing is a dynamic process, restoring lost or damaged skin layers through the activity of cytokines, growth factors, extracellular matrix molecules and stimulation of various cell types.3 Early in the healing process, initiation of re-epithelialisation is a critical step in the closure of the wound.4 The basic cellular and molecular mechanisms that result in effective wound healing involve four phases,2 namely;
The role of insulin in wound healing process
Insulin is a peptide hormone with multiple physiological roles. It regulates blood glucose levels and is known to have a beneficial role in wound healing. Since insulin can potentially help restore the integrity of damaged skin, it is of interest in the field of wound repair, particularly owing to its low cost relative to other growth factors, and, thus, is more likely to be considered for incorporation into wound dressings, bio adhesive films and hydrogels.9
The beneficial effects of insulin in wound repair have been observed over many decades, and can be traced back to the early 20th century, to surgeons who found differences in the postoperative wound healing amongst diabetic and non-diabetic patients.9 In the former group, wounds would be more likely to fail to re-epithelialise normally, making them susceptible to infection. As any ensuing infection spread to the systemic circulation, further complications arose. Thalhofer, the first to report insulin use as a means to treat postoperative (non-diabetic) acidosis10 had been monitoring three patients who had undergone surgery and were suffering from severe vomiting and post-operative metabolic imbalance. This work showed that there was strong evidence linking acidosis with the development of severe infection and death. After injecting three patients with glucose and 10 units of insulin, the symptoms of acidosis disappeared and the metabolic balance was restored. Improved recovery for patients was supporting evidence for the role of insulin in regulating tissue repair. Foster11 was also attempting to treat similar cases of diabetic acidosis by administering 30-75units of insulin.
The mortality rate from infection in diabetics was decreased from 40% to 12% in those patients receiving insulin therapy intravenously amongst 20 patients. Schazzilo & Ksendowsky used 1 unit of crystalline insulin, injected into test animals, to investigate the effects of insulin on bone fracture repair. Results showed a faster rate of healing compared to controls.12 Joseph13 administered 10 units of insulin once daily to five patients for the treatment of non-diabetic bed sores. Results from this study demonstrated wound healing of between 50% to nearly 100% after 14 days of therapy. Stuck14. studied the effect of intravenous administration of 1.5units insulin to promote faster bone healing in rabbits with fractured left fibulas. Faster calcification of the callus in the presence of insulin was reported after 14 to 28 days when compared to the control group, whilst maintaining the same bone morphology in both the insulin and control groups.
Despite the considerable amount of evidence over many decades demonstrating stimulation of healing and reductions in the time required for wound repair, the underlying mechanism of insulin-induced healing is not entirely clear. A variety of cell and molecular approaches have been employed to determine the effects of insulin on cutaneous wounds and have shown that insulin can stimulate keratinocyte migration in a dose and time dependent manner, acting in an insulin-receptor-dependent but EGF/EGF-R-independent manner. Conversely, the ability to stimulate both the insulin and IGF-1 receptors may broaden the applicability of insulin in different wound types, particularly when one receptor may be dysfunctional (e.g. in type II diabetes). Understanding the process by which insulin accelerates the wound closure is important because it will provide insight into many potential applications of the healing process.
Research consistently highlights the importance of the insulin receptor, a transmembrane molecule,activated by insulin, IGF-I and IGF-II. It belongs to a large class of tyrosine kinase receptors found in all cell types, including keratinocytes and fibroblasts.15 Liu, et al.16 showed that topical application of insulin to excision wounds stimulates keratinocyte migration. This migratory enhancement involves the PI3K-Akt pathway, and identifies Rac1, a small GTPase, as a molecule activated downstream of PI3K-Akt.17 Activation leads to translocation of Rac1 to the plasma membrane, followed by activation of Rac1 substrate, the integrin α3 and the extracellular matrix molecule laminin332.18 The effects of insulin on keratinocyte migration led to propositions that insulin-accelerated wound healing involves increased expression of the integrin α3β1 in keratinocytes as well as an increase in the levels of LN332.
The latter molecule is a matrix protein secreted by migrating keratinocytes at the leading edge,19 where it mediates keratinocyte polarity and cell migration.20 After a traumatic skin event, quiescent epidermal keratinocytes are activated and express α6β4 and α3β1 integrins, which control their migration on LN332 and facilitate the development of the basal membrane. So, insulin stimulates keratinocyte integrin α3 expression and LN332 deposition, and that suppression of these proteins in vitro or in vivo inhibits insulin-induced keratinocyte migration and wound healing, strongly suggesting a critical role for these molecules in the action of insulin to stimulate healing. However, the relationship between LN332 and cell migration remains controversial. Some studies have implicated LN332 in inhibiting cell migration,21 whereas others support a role for LN332 in promoting keratinocyte migration.18
Expression of the insulin receptor, IRS-1, IRS-2, ERK and Akt are increased in the tissue of wounds compared to intact skin, suggesting that the insulin signaling pathway may have a critical role in this process. Akt, in particular, can phosphorylate proteins that regulate cell survival, lipid and glycogen synthesis.22,23s Recently, data demonstrate that Akt activation is an important step for VEGF release in skin wounds, through a post-transcriptional mechanism in keratinocytes,24,25 and is necessary for vascular maturation and angiogenesis during cutaneous wound healing.26 These various pathways are inhibited in the injured skin of diabetic rats and correlate with a delay in the time required for complete wound healing. Lima et al.8 showed that insulin signaling pathways are promoted in the injured skin of normal rats, whereas these pathways are attenuated in diabetic animals due to insulin deficiency. However, when injured skin of diabetic rats is treated with a topical insulin cream, an acceleration of wound healing occurs, together with a recovery in the proteins of the insulin signalling cascade.27 Therefore, expression of proteins involved in the early phase of insulin exposure, namely, IRS-1,2 and Akt, are increased in healing tissue when compared to healthy skin.
Insulin stimulation of ERK involves the tyrosine phosphorylation of IRS proteins, which in turn interact with the adapter protein, Grb2 (growth factor receptor-bound protein-2), recruiting SOS exchange protein to the plasma membrane for activation of Ras (one member of a large family of small molecular weight GTP-binding proteins).28 Once activated, Ras acts as a molecular switch, stimulating a serine kinase cascades through the stepwise activation of Raf, MEK (protein kinase that activates MAP kinases) and ERK. Activated ERK can translocate into the nucleus, where it catalyses the phosphorylation of transcription factors, stimulating a transcriptional program that leads to cellular proliferation or differentiation.29 The protein levels of ERK are increased in injured skin, suggesting that the ERK signaling pathway can also play a direct role in the regulation of cellular growth and differentiation. It is important to emphasise that ERK activation is necessary for keratinocyte pro-migratory signaling pathways.30,31 It is now well established that an increase in the migration of endothelial progenitor cells from bone marrow to wounded skin accelerates wound healing.8 The regulation of this process is complex and involves activation of eNOS in the bone marrow by VEGF that is produced in the site of injury, enhancing the mobilisation of endothelial progenitor cells, which are recruited to the cutaneous wound site by an increase in tissue levels of SDF-1α.22,32 Insulin signaling pathways are summarized in Figure 2, which shows the effect of insulin on cellular and molecular mechanisms involved in wound repair.
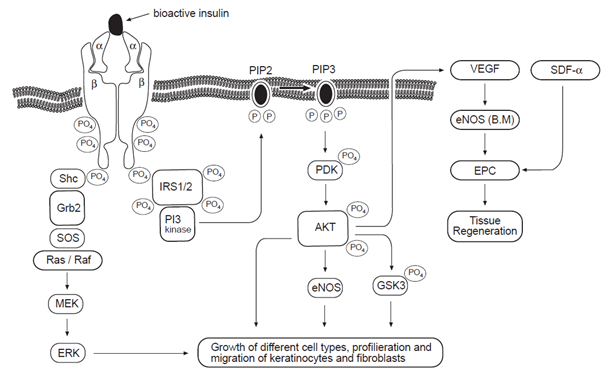
Figure 2 Effect of insulin on cellular and molecular mechanisms of wound healing. Insulin induces activation of IR/IRS/PI3K/AKT pathways. On the upper right-hand side, AKT is shown to increase VEGF that will induce the phosphorylation and activation of eNOS in bone marrow, with consequent mobilization of EPCs to the circulation, where they participate in tissue regeneration.
Other proposed mechanisms of action for insulin in wound healing exist, such as the enhancement of expression of neutrophil adhesion molecules to reinforce the cellular functions of migration, phagocytosis and bactericidal actions.33 Although neutrophils act as traumatic scavengers and help wound healing in some ways, neutrophils also have a negative impact on wound healing, particularly when excess or hyper-functional neutrophils accumulate in the wound environment. Suppression of neutrophil infiltration could promote wound healing. Chen et al.34 provided observations regarding the number and function of neutrophils with or without low-dose topical insulin and a preliminarily investigation of the underlying mechanisms. It was shown that topical insulin application decreases neutrophil infiltration by inhibiting MIP-2 expression and advanced neutrophil resolution. So, insulin regulates the inflammatory response during wound healing and findings support local and systemic administration of insulin could be an effective treatment for skin wound incisions.35
Pharmaceutical applications of insulin in wound healing
The dermatological properties of insulin have been studied for almost a century.13 Rosenthal demonstrated that topically applied insulin increases wound tensile strength and decreases healing times in Wistar rats.36 Similarly, Udupa and Chansouria applied a linear musculo peritoneal wound in rats and administration of 2units of insulin per 100g subcutaneously led to faster wound healing in insulin-treated rats when compared to a control group.
Histological analysis of wound tissue showed an earlier appearance of collagen fibres with more dense and well-oriented morphology versus control animals.37 Recently, Zhang et al.35 tested local insulin-zinc injections on skin donor sites of rabbits. Subcutaneous injections of 0.25units of long acting insulin-zinc suspensions were directly administered to the backs of dermatomed adult rabbits. Insulin was injected every other day and compared to controls consisting of placebo and zinc suspension alone. With localised injection, wound healing was accomplished in 9 days, significantly faster than any of the control groups and without signs of major systemic side effects.
Lima et al.8 investigated the effect of a topical cream, containing insulin, showing that it accelerated wound healing and induced a rescue in the levels of tissue proteins involved in the early steps of insulin action, as mentioned above.8 Topical application of this insulin-containing cream (0.5U per g cream, University of Campinas, Brazil; patent number PI 0705370-3) was shown to normalise the wound healing time in diabetic animals. Furthermore, the treatment also increased expression of other relevant proteins, such as eNOS, VEGF and SDF-1α. In an extension to this study, the same authors applied their novel cream to diabetic patients and clinical evaluation demonstrated markedly improved wound healing. This supported the possibility of a promising and cost-effective method for treating this serious complication of diabetes.8 In a similar study, Achar et al.38 showed that topical use of a cream containing insulin-like growth factor (IGF-1) improves wound healing in both diabetic and non-diabetic animals, with increased expression of my fibroblasts.
Recently, an insulin spray-based formulation has been used successfully to treat patients with diabetic ulcers.18 Ulcers were cleaned with normal saline and then irrigated with 4units (0.1ml) of human soluble insulin (Actrapid®) in 1ml normal saline (0.9%) for each 10cm2 of wound area. The solution prepared was sprayed on the ulcer surface with an insulin syringe twice daily and the ulcer was left to dry and covered with sterile cotton gauze.39 Furthermore, insulin has been used to treat burns in humans, rats18 and rabbits35 with encouraging outcomes. In addition to the in vivo studies, results from experiments on in vitro cultured cells have shown that insulin increases the rate of growth of fibroblasts, cells that are critically involved in the development of the granulation tissue, suggesting that insulin can act as a growth hormone.40
Human recombinant crystalline insulin can be efficiently encapsulated into PLGA (D,L;50:50,5-15kDa) microspheres using a solid-in-oil-in-water (S/O/W) emulsion solvent evaporation technique. Insulin-PLGA microspheres have spherical and discrete morphology and their mean particle size ranged between 130-160µm. Further data showed that insulin was released for 25days in a controlled manner. High level of insulin bioactivity over a 23-day release period was demonstrated by stimulating cell signalling responses in Rat L6 myoblasts. Furthermore, an in vitro scratch assay in HaCat cells established that insulin released from PLGA microspheres stimulated rapid cell migration following an induced scratch. Hrynyk et al.41 suggested that crystalline insulin encapsulated within PLGA microspheres offers potential for long-term and controlled delivery of bioactive insulin for topical delivery devices and could have significant clinical applications.
The routine clinical use of insulin in wound management is not a generally accepted first-line treatment option. Systemic insulin administration has limited applicability because of significant side effects, including hypoglycemia, hypokalemia and hypoaminoacidemia.42 The doses of topical insulin that have been studied vary from 5 to 15units in mice to 20 units in horses. Administration techniques can be crude, with drops of insulin solution used in rats and humans.43 Topical insulin application, although optimal in theory, has been hindered by the lack of a vehicle that can deliver insulin reliably to the wound bed at a controllable rate.35 Formulation of sophisticated delivery systems, such as insulin-loaded particulate systems in optimized vehicles (adhesive films or hydrogels) is a promising development to ensure controlled delivery into wound environments.
None.
The author declares that there is no conflict of interest.

©2016 AbdelKader, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.