Journal of
eISSN: 2473-0831


Research Article Volume 5 Issue 1
Correspondence: Jamilu Muazu, Department of Pharmaceutics and Pharmaceutical Microbiology, University of Maiduguri, Nigeria,, Tel 8037439543
Received: October 31, 2016 | Published: June 12, 2017
Citation: Igbokwe N, Madu SJ and Muazu J (2017) The Effect of Yoyo Bitters on the Dissolution of Metformin Tablets. J Anal Pharm Res 5(1): 00130. DOI: 10.15406/japlr.2017.05.00130
Background: Yoyo bitters is an oral poly herbal liquid preparation popularly marketed and used in Nigeria. Its use is rapidly increasing among patients with chronic diseases such as diabetes and hypertension. This is because it has been claimed to regulate blood pressure, control body weight, and facilitate digestion among others. Metformin is an oral anti diabetic agent. Both the incidences of Diabetes and use of Yoyo bitters are increasing hence setting the stage for possible co-administration of these two agents. This also increases the potential for Herb-drug interaction. The aim of the study is to investigate the effect of Yoyo bitters on the dissolution of metformin tablets.
Methods: Physicochemical tests (Weight uniformity, Crushing strength, Friability and Assay) were carried out on two different brands of metformin tablets and physical properties of the Yoyo bitters were also determined. The in-vitro dissolution of metformin tablets with and without Yoyo bitters in dissolution media was then carried out at pH 1.2, 4.5 and 6.8 using United States Pharmacopoeia (USP) apparatus 1 (basket) at 100rpm with 900ml of the dissolution medium (sink condition) maintained at a steady temperature of 37±0.5ºC. Two different brands metformin 500mg immediate release tablets were used and the analysis was done using UV-Spectrophotometer at a wavelength of 232nm.
Results: The results obtained show that at pH 1.2, the percentage amount of metformin released decreased significantly in the presence of Yoyo bitters for both brands (p=0.002 and p=0.013 respectively). At pH 4.5 there was no significant difference in metformin released for the Diabetmin® (p=0.196) and a significant difference was observed with Sandoz® (p=0.045). At pH 6.8, a significant decrease in percentage metformin released in the presence of Yoyo bitters was observed for both brands (p=0.011 and p=0.044 respectively).
Conclusion: Yoyo bitters decreased the dissolution of metformin tablets at pH 1.2, 4.5 and 6.8. Due to therapeutic failure, toxicity etc caused by decreased dissolution, concomitant use of metformin and Yoyo bitters should be discouraged.
Keywords: yoyo bitters, metformin, dissolution, diabetes
The World Health Organization (WHO) defined herbal medicine as: ‘any part of a plant in which one or more of its parts can be used for therapeutic purposes or as precursors for the synthesis of useful drugs. Herbal medicines include herbs, herbal materials, herbal preparations and finished herbal products that contain parts of plants or other plant materials as active ingredients.1 Prior to the advent of modern medicines, use of herbal remedies was the only available treatment known to mankind hence the use of herbal remedies for several decades by people from diverse cultures. Based on a report of WHO global survey, the use of herbal medicine throughout the world has exceeded the use of conventional therapies by two to three times. While 90% of the population in Ethiopia use herbal remedies for their primary healthcare, surveys carried out in developed countries like Germany and Canada show that at least 70% of their population have tried complementary or alternative medicine at least once.2 About 25% of drugs prescribed worldwide are derived from plants.3
Herbal bitters are blends of herbs, spices, roots, seeds and bark used as a tonic to aid in digestion, particularly of heavy fatty meals. Medicinal herbal bitters on the other hand contain blended ingredients in a water or alcohol base used for the treatment of illnesses. Bitters contain complex carbohydrates, secondary metabolites, vitamins and minerals that have antioxidant, antiviral and other pharmacological properties. They are used to aid digestion and metabolism, relieve occasional heartburn, calm upset stomach and nausea, increase absorption of fat soluble vitamins, help maintain healthy blood sugar levels, ease constipation and regulate bowel movements, help mop up free radicals and stimulate immune function among others.4 Herbal bitters are used all over the world, some notable examples of bitters available today include: Alomo Bitters (Ghana), Amaro Montenegro (Italy), AmerPicon (France), Aperol(Italy), Araucano (Valparaíso, Chile), Averna(Italy), Balsam (Eastern Europe), Becherovka (Czech Republic), Beerenburg (Netherlands), Blutwurz(Bavaria), Calisaya (USA), Campari (Italy), FernetBranca (Italy), Fernet Stock (Czech Republic), Gammel Dansk(Denmark)Jägermeister (Germany), Jepson’s (United States), Killepitsch (Düsseldorf, Germany), Kuemmerling (Germany), Pelinkovac (Balkans), Quinquina (France - originally from South America), Ramazzotti (Italy), Ratzeputz (Germany), Riga Black Balsam(Latvia), Schierker Feuerstein (Germany), Schwartz hog (Germany), St. Vitus (Germany), Sirop de Picon (France), Suze (France), Underberg (Germany), Unicum (Hungary), WódkaŻołądkowaGorzka (Poland), Wurzelpeter (Germany).
Yoyo Bitters, a typical example of a commercially marketed herbal bitter, is an oral preparation popularly used in Nigeria which is contained various parts of plants such as Aloe vera, Cinnamum aromaticum, Citrus aurantifolia, Acinos arvensis, and Chenopodium murale.5 Its use is common among patients with chronic diseases like hypertension and diabetes.6
Herb-drug interaction refers to the possibility of an herbal drug altering the bioavailability or clinical effectiveness of a conventional drug when given concurrently.7 Since herbal medicines contain more than one pharmacologically active ingredient and are commonly used with many prescribed drugs, there are potential herb-drug interactions. The concurrent use of herbal and drug combinations may raise the potential of drug- herb interactions as observed in some studies. Ginkgo biloba was reported to cause bleeding with warfarin and acetylsalicylic acid.8 Hypericumperforatum (St. John’s wort) has been reported to decrease plasma concentrations of cyclosporine, tacrolimus, oral contraceptives, simvastatin, nifedipine, and verapamil.9 Both the incidences of chronic diseases (hypertension and diabetes) and the use of herbal bitters are increasing, thus, setting the stage for potential co-administration of antihypertensive/ anti diabetics and herbal remedies. The aim of the study is therefore, to investigate the effect of Yoyo bitters on the dissolution profiles of metformin tablets.
Metformin tablets (Diabetmin® and Sandoz®) were purchased from pharmacy unit of University of Maiduguri Teaching Hospital, Yoyo bitters was purchased from a registered retail pharmacy, Sodium hydroxide (BDH chemicals, Poole, England). Hydrochloric acid (BDH chemicals, Poole, England). Potassium dihydrogen orthophosphate, Acetic acid, Sodium acetate monohydrate, Weighing balance (Sartorious, Germany), PH meter (Jenway, Japan), Hardness Tester (Erweka Germany), Friability Tester (Erweka, Germany), Dissolution Tester (Erweka DT-700, Germany), 6405 UV/Vis. Spectrophotometer (Barlowood scientific, UK).
Uniformity of weight test of metformin tablets
The USP method was adopted, twenty tablets were randomly selected from each brand and weighed individually. The mean weight of the tablets was then calculated and the standard deviation was determined.
Crushing strength for metformin tablets
The Erweka hardness tester (TBH 100, Germany) was used in measuring the crushing strength of the tablets. Six (6) tablets were randomly selected from each brand; each of these tablets was in turn placed between the anvil and the spindle of the hardness tester and subjected to increasing pressure by turning the knurled knob in a clockwise direction at constant rate until the tablet was crushed. The value of the pressure applied at this point gives a measure of the hardness. The mean and standard deviation of the six determinations was then taken for each sample.
Friability test for metformin tablets
The USP method was adopted, ten tablets were randomly picked from each brand and weighed accurately. They were then placed inside the drum of friabilator (Erweka, Germany) and operated for 4min at a speed of 25rpm. Thereafter, the intact tablets were removed from the drum, dusted and weighed. The percentage loss in weight was calculated and recorded as friability value for that sample.
Assay of metformin tablets
The USP method was adopted with slight modification, five tablets were weighed and powdered. A quantity containing 0.1g of metformin was shaken with 70ml of water for 15min, it was diluted to 100ml with distilled water and filtered. The first 20ml was discarded, 10ml of the filtrate was diluted with 100ml water and 10ml of the resulting solution was further diluted to 100ml with water to obtain 10µg/ml. The absorbance was measured at a wavelength of 232nm using UV spectrophotometer (Barlowond scientific, UK).
Physicochemical characterization of yoyo bitters
The colour of Yoyo bitters was determined by visual inspection of the liquid preparation and recorded. The taste and pH of the herbal product were also determined.
Dissolution time test
The USP method was adopted, the dissolution medium was prepared as described earlier,7 300ml of Yoyo bitters was measured in a 1000ml measuring cylinder, and 0.1N HCl was added to make 1L of the dissolution medium. The media without yoyo bitters was 0.1N HCl, for the dissolution test 900ml was used while the remaining volume was used to maintain sink conditions. The same procedure was repeated for acetate buffer and phosphate buffer. The 0.1N HCl and acetate buffer were prepared according to USP while the phosphate buffer was prepared according to the British Pharmacopoeia (BP) method. In vitro dissolution test was carried out using tablet dissolution test USP apparatus 1 (basket). The dissolution test was carried out in three media without Yoyo bitters: 0.1N HCl at pH 1.2, acetate buffer at pH 4.5 and phosphate buffer at pH 6.8; and three media containing Yoyo bitters. The temperature of the dissolution medium was maintained at 37±0.5°C at a speed of 100 rpm. A tablet was placed in each vessel and the equipment operated for 60min. Sample aliquots of 10ml were taken at 5,15,30,45 and 60min and replaced with 10ml of fresh dissolution medium to maintain sink conditions. The experiment was done in triplicate. Each of the withdrawn samples was diluted to 100ml; 10ml of the resulting solution was taken and further diluted to 100ml then filtered through a 0.45µm membrane filter discarding the first 20ml filtrate. The filtrate was then analyzed at a wavelength at 232nm.10 using UV-VIS spectrophotometer. The experiment was carried for the two brands of metformin tablets in media with and without yoyo bitters at pH 1.2, 4.5 and 6.8.
Data analysis
SPSS version 16.0 was used in the analysis of the data using paired t-test at a level of significance of p<0.05.
Table 1 shows the physicochemical parameters of the metformin tablets. Table 2 represents the physical properties of Yoyo bitters. Dissolution of a drug in solid dosage form into an aqueous medium is critical for its absorption, which in turn has an impact on its bioavailability and therapeutic efficacy. Variables such as weight uniformity, hardness, friability and percentage purity also play important roles in the efficient release of the drug for systemic absorption.11 These parameters were therefore carefully analysed before carrying out the dissolution studies. The results obtained from weight uniformity and friability tests (Table 1) conformed to the specifications in the United States Pharmacopoeia.12.
|
Parameter |
Observation |
|
|
Diabetmin® |
Metformin Sandoz® |
|
|
Shape |
Round |
Round |
|
Colour |
White |
white |
|
Embossment |
HD |
M 500 |
|
Uniformity of weight (g) |
0.56±0.06 |
0.52±0.05 |
|
Crushing strength (KgF) |
11.75±1.52 |
13.41±2.61 |
|
Friability (%) |
0.02 |
0.02 |
|
Assay (% purity) |
97.47±6.33 |
95.32±3.11 |
Table 1 Physicochemical Parameters of Metformin Tablets
The pH of the Yoyo bitters was 3.4 (Table 2) which showed that it is acidic in nature. This could be due to some of its constituents such as Cinnamon aromaticum which contains Cinnamic acid, Citrus aurantifolia containing Citric acid and Ascorbic acid and Acinosarvensis which contains Linoleic acid.13 A research by Olubunmi et al.7 recorded the pH of the Yoyo bitters used as 5.8. This variation in pH could be due to batch to batch differences mostly caused by poor adherence to strict quality control measures. A study.14 on chemical 0 and alkaloids has been known to be responsible for the bitter taste observed in many foods and herbs. This can probably account for the bitterness of Yoyo bitters.
|
Parameter |
Observation |
|
Color |
Brownish black |
|
Taste |
Bitter |
|
pH |
3.4 |
Table 2 Results of Physical Properties of Oral Liquid Yoyo bitters
Figures 1-3, illustrates the dissolution profiles of metformin alone and with Yoyo bitters at pH 1.2, 4.5 and 6.8 respectively. From the graphs it can be seen clearly that there is evidenced reduction in the dissolution profile of metformin due to the presence of Yoyo bitters at all pH levels.
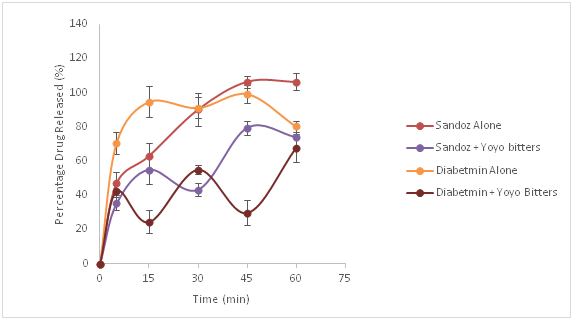
Figure 1 Dissolution profiles of metformin (Diabetmin® and Sandoz®) with and without Yoyo bitters in media at pH 1.2. (p< 0.05).

Figure 2 Dissolution profiles of metformin (Diabetmin® and Sandoz®) with and without Yoyo bitters in media at pH 4.5.
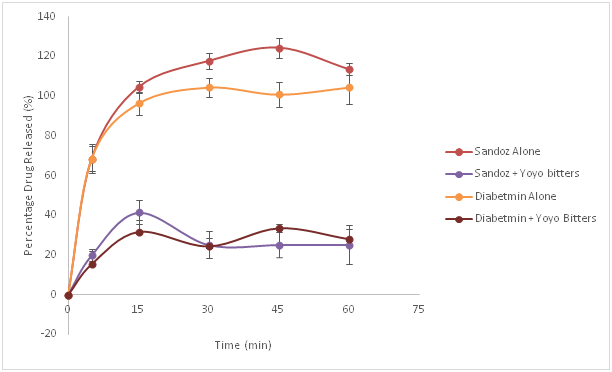
Figure 3 Dissolution profiles of metformin (Diabetmin®and Sandoz®) with and without Yoyo bitters in media at pH 6.8. (p< 0.05).
Figures 1-3 show the dissolution profiles of metformin tablets alone and in the presence of Yoyo bitters at pH 1.2, 4.5 and 6.8.
The results obtained from the experiment showed that for both brands of metformin tablets, at pH 1.2, which mimics the acidic nature of the stomach, the percentage release of metformin without Yoyo bitters conformed to the BP.15 standard which states that, 70% drug should be released within 30min. This means that the two brands of metformin (Diabetmin® and Sandoz®) passed the dissolution test at this pH but in the presence of Yoyo bitters, a decrease in the percentage release of metformin was observed for both Brands. This implies that the tablets did not pass the dissolution test in the presence of Yoyo bitters as not up to 70% 0f the drug was released in both cases.
The paired t-test for metformin in media with and without Yoyo bitters for Diabetmin® and Sandoz® showed a significant difference (p=0.002 and p=0.013 respectively) in percentage release of metformin. At pH 4.5, the percentage metformin alone released for both brands of metformin used in the experiment conformed to the specification in the monograph of metformin as stated in the BP (2009). In the presence of Yoyo bitters, the percentage release of metformin decreased but the decrease with Diabetmin® is not significant (p=0.196) whereas that of Sandoz® is statistically significant (p=0.045). This pH mimics the transit between the stomach and small intestine.
At pH 6.8 which is the pH of the small intestine, both brands of metformin alone also conformed to the monograph of metformin USP. With Yoyo bitters, the percentage metformin released was lower than that seen in metformin without Yoyo bitters; hence the tablets did not pass the dissolution test in the presence of Yoyo bitters as not up to 70% of the drug was released within the stipulated time frame. The difference in percentage release is statistically significant as observed with Diabetmin® (p=0.011) as well as with Sandoz® (p=0.044).
Yoyo bitters probably impaired the solubility of metformin either by chelation or by forming insoluble complexes, thereby hampering the release of metformin. This could possibly account for the change in the dissolution profiles in its presence. Analysis of Yoyo Bitters as reported by Olubunmi et al.7 eluted a number of substances that could not be identified. These constituents could have interacted with the process of dissolution at all the stages. Again, Yoyo bitter is a mixture of about five herbs, Aloe vera inclusive. A report by Chavez et al.16 stated that herbs like Aloe vera and other anthraquinone-containing herbs could inhibit absorption of conventional drugs as a result of chelation or formation of insoluble complexes with the drugs.
The absorption of metformin takes place primarily in the small intestine,17 therefore the dissolution observed at pH 6.8 is critical to the absorption or bioavailability of the drug as this pH mimics the small intestine. This is of huge clinical relevance as the decrease in dissolution of metformin tablets could lead to a decrease in absorption of the active ingredient and in turn bioavailability of the drug. Decreased bioavailability can result to decreased concentration at site of action hence treatment failure resulting to poor glycaemic control, diabetic complications such as uncontrolled hypertension, posing a threat to some organs of the body like the eyes, heart, brain and kidneys thereby compromising positive clinical outcomes in patients taking such medications. Concurrent use of Yoyo bitters and metformin tablets can increase the potential for herb–drug interaction, which may have significant clinical consequences.
The two brands of metformin tablets passed the quality control parameters. Yoyo bitters decreased the dissolution of metformin tablets at pH 1.2, 4.5 and 6.8 which may affect efficacy, safety etc and therefore, concomitant use of the metformin and Yoyo bitters should be discouraged.
None.
The authors declare no conflicts of interest related to this article.
None.

©2017 Igbokwe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.