Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 6
Correspondence: Victor O Fadipe, University of Zululand, Department of Chemistry, Private Bag X1001, KwaDlangezwa, 3886, South Africa, Tel 035 902 6099, Fax 035 902 6568
Received: April 15, 2016 | Published: July 14, 2016
Citation: Fadipe VO, Rojas JAY, Areola ES, Badillo JJ, Abdallah HH, et al. (2016) Synthesis, QSAR Model Study and Antimicrobial Evaluation of Esters and Thioester Derivatives of Isonicotinic Acid on the Different Strains of Mycobacterium Tuberculosis. J Anal Pharm Res 2(6): 00037. DOI: 10.15406/japlr.2016.02.00037
Isoniazid is one of the widely used first-line drugs in the treatment of tuberculosis for many years but the mechanism of the action of the drugs is still not clear. Herein, we report the synthesis, QSAR model study and biological effects of esters and thioester derivative of isonicotinic acid (INA) on different strains of Mycobacterium tuberculosis in an attempt to establish the mode of action of the active form of Isoniazid (INH). The esters of INA are expected to show antibiotic activities against strains of Mycobacterium tuberculosis upon activation by intracellular nonspecific esterases, while thioesters is expected not to show antimicrobial effects and thus served as control. The obtained results indicated that the synthesized esters did not show antimicrobial activity. The conclusion drawn from the result is that either INA is not the active form of INH or that the esters show poor water solubility- in addition to the possibility of acquired during resistance of the strains during culture and testing - all of which could hamper mycobacterial cell up take. This suggests that more studies are needed to search for the possible active form of isoniazid.
Keywords: oxidation, Mycobacterium tuberculosis, isoniazid, rifampicin, pyrazinamide, ethambutol, streptomycin; thioester, micro-organism, isonicotinic, hydrolyzed, prodrug
Mycobacterium tuberculosis (Mtb) was discovered in 1882 by Robert Koch.1 Tuberculosis is caused by this bacillus and it is widely spread in the world. It has been reported to be a common opportunistic infection of HIV. Isoniazid (INH) is used for the treatment of tuberculosis like other first line drugs (isoniazid, rifampicin, pyrazinamide, ethambutol or streptomycin). Isoniazid is a pro-drug that is activated by KatG enzyme, and its products inhibit the production of nicotinic acids generated in the InhA complex. KatG enzymes are limited to Mtb.2
Due to the high resistance of Mtb in the hospitals caused by inappropriate treatment administration, it has become necessary to search for isoniazid’s derivatives that are probable intermediate responsible for its biological activity. This probable intermediate which is synthesized derivatives is therefore expected to be potent enough to kill this micro-organism (Mtb). Ester and thioester are the active forms of isonicotinic acid (INA) that can be generated under different condition (Figure 1).3
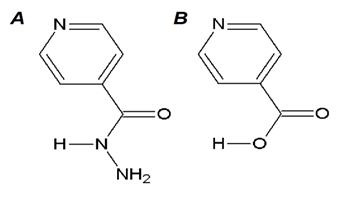
Figure 1 Structural drawings. (A) Isoniazid (INH) is a first line drug in the treatment against tuberculosis. (B) Isonicotinic acid (INA) could be the possible active form of INH as a postulated prodrug generated by KatG. Both molecules were drawn using Chem Sketch.3
The mechanism of action of isoniazid against Mtb has been studied from diverse approaches.4 The in silico analysis suggests that the mechanism of action can either be accomplished by free radical generation,5 or possibly accomplished by redox reaction mechanism.5 The latter assumption seems to agree with the experimental results. Consequently, our study focuses on the mechanism of action of INH and INA. It is possible that the isonicotinic acid and its derivatives, esters and thioesters, may also be active. When these derivatives; esters or thioesters are hydrolyzed, either of the compounds can easily liberate its acid free form.
The hypothesis of the mechanism of action of Isoniazid by free radical had been reported earlier (Figure 2 in 5). The INH is activated by an intracellular enzyme of Mtb, KatG, which has a hemo group with an iron forming the complex I. The iron has +4 oxidation state (Fe+4), but the ground state of iron shows anoxidation state +3, which precedes the iron state seen on the complex (I). It interacts with hydrogen peroxide and converts it to a molecule of water and the compound I. Compound I is then bonded to an oxo group and an iron atom to make it stable (see also Figure 2 in 5).
The generated INH is in the radical state, nitrogen at one end, which is used to form the complex II which has +3 oxidation states in iron atom. Only Fe+3and water are created after this complex has gained an electron. With that, the generated cycle of one radical in the INH is closed. Since a second radical is the INH radical, it is then transformed by a radical attack on the previous nitrogen, and a second radical, that is named Nicotinyl radical, is then liberated.6–9
The hypothesis of the mechanism of action of isoniazid by an ionic pathway also creates an intracellular isonicotinic acid which occurs only inside of the mycobacterial cell (Figure 2 in 5).
The Figure 2 in 5 shows an Mtb, since the prodrug of INH enters into the mycobacterium, and KatG activates the alleged prodrug, the product of the activation is the isonicotinic acid. It will be in a deprotonated form and at that stage; the pH determines its behavior. If it enters the Mtb cell plasma with its neutral pH and pKa value of 4.8, then its intracellular concentration will increase because the neutral compound becomes a negatively charged acyl anion. Hence, it cannot get out by passive diffusion; it is trapped inside the Mtb cells. Consequently, if there is the possibility to generate a substitution of nicotinic acid and to form the iso-NAD complex, which inhibits InhA activity, letting the cell without the production of mycolic acids.4,10–14
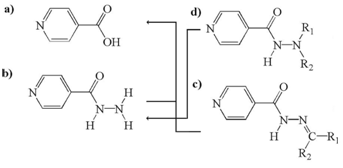
Figure 2 Chemical variety of basic molecules and the metabolites which can occur either by enzymatic activities or “nonenzymatic” hydrolysis: a) INA, b) INH, c) Hydrazones, and d) Hydrazines.15
The findings from the literature indicated that the active form of the INH could be the a fore mentioned INA.5,15,16 According to this hypothesis, esters and thioesters of the INA are expected to have biological activity against Mtb strains when they are activated by non-specific intracellular esterases. In support of this supposition, we carried out the following combined approach to gain insight into the intriguing mechanism of action of INH: (i) created a computational model, (ii) used activity data to generate QSAR models, (iii) carried out the synthesis of esters and thioesters of INA, (iv) before the determination of the antimicrobial effects of these compounds in Mtb strains.
Synthesis of ester and thioester of isonicotinic acid
The solvents used in the experiments showed technical purity (Fermont and Baker). The reagents had analytical purity (Sigma-Aldrich). The measurements of NMR of 1H and 13C were carried out on a Varian Gemini spectrometer at 200MHz for 1H and 50.3 MHz for 13C. The solutions with deuterated chloroform contained tetramethylsilane (TMS) as an internal reference; while the infrared data was collected on a Varian-00 FT-IR (Scimiltar Series) spectrometer; in addition to the ultraviolet data which was obtained from a Varian Cary-100 ultraviolet spectrometer.
General procedure for the preparation of esters of isonicotinic acid
A solution of isonicotinic acid (0.010 mol), alcohol (1-butanol, 1-hexanol, 1-octanol) (0.015 mol) and H2SO4 underwent reflux for 4 hours with magnetic stirring.17 The reaction was monitored and verified to completion by TLC; it was cooled and neutralized with a saturated solution of NaHCO3. After then, the product of the reaction was extracted with EtoAc (3x50 mL), the organic phase was washed with water and dried with Na2SO4 anhydride; the organic phase was filtered and evaporated to dryness in rotary evaporator. The product was purified again using column chromatography, using hexane initially and then increasing its polarity with chloroform as mobile phase.
General procedure for the preparation of thioesters of isonicotinic acid8–10
A solution of isonicotinic acid (0.010 mol), thionyl chloride (0.012 mol) and chloroform (10mL) was heated at 70°C for 9 hrs, and the components were mixed by magnetic stirring under N2 atmosphere, and a solution of thiol (0.015 mol) in chloroform (5mL) was added.18 The reaction was monitored and verified to completion by TLC. The mixture was cooled and neutralized with a saturated solution of NaHCO3, and was finally extracted with ethyl acetate (3 times 50 ml EtOAc). The organic phase was washed with water, followed with a solution of 10% NaOH and was dried with anhydrous Na2SO4. In the next step it was filtered; subsequently, it was evaporated to dryness on the rotavapor. The product of the reaction was purified on column chromatography with silica gel, using hexane initially and then with chloroform in order to increase the polarity of the mobile phase.
Infrared spectroscopy
The infrared spectrometer is expected to provide information about two important bands: the O-C=O stretching of an ester and the S-C=O stretching of a thioester. The typical infrared signal of a C-O group of an ester is fairly different from the C-S signal of a thioester because the mass and electro-negativity of the oxygen and sulfur are quite different. Sulphur atoms are heavier in mass than oxygen atoms. Hence, an increase in mass lowers the observed frequencies. A more electronegative atom like oxygen destabilizes the resonance structure of the ester, giving the C=O a higher bond order, that is a stronger double bond character. As a general result, the ester signals are absorbed at a higher frequency. Because the sulphur is far less electronegative than oxygen, the former has a more stabilized resonance structure due to its greater ability to donate its electrons, which in general then results in lower frequencies.
The stretching vibration due to vibrational intensity of C=O bond shows greater frequencies between 1750 and 1735 cm-1(less wavelength) than ketones frequencies.
The IR signals of the C-O stretching are basic 2 coupled asymmetric vibrations: C-C (=O)-O. The observed range is 1300-100 cm-1 while the ester saturated bands strongly appeared in a region around 1210 to 1163cm-1. The corresponding frequencies for vinylic esters and phenolic moieties were observed near1190 to 1140cm-1. The O-C-C signals of esters coming from primary alcohols were observed around 1164 to 1031cm-1. Ester derivatives of secondary alcohols had a distinct signal at 1100cm-1. Aromatic ester derivatives of primary alcohols showed a distinct signal at 1111cm-1.19,20
NMR spectroscopy
The signals of 1H- NMR for alkanes oscillate generally between 0.8 and 4.0δ, while the aromatic compounds showed displacements superior to 7.0δ.21,22 The 13C-NMR provided the C-C scaffold structure of each compound.
Anti-biograms
The experimental data were generated from the modified proportions of dilutions for a slow growing mycobacterium. Using this method, the susceptible assay can be made directly and indirectly. Usually, the pure cultures of organism were made in the indirect form where it was grown in solid media, 7H10 or 7H11 Middle brook agar or Löwenstein-Jensen media.23
Agar preparation
Water bath was heated to a temperature of 50-56°C, then into an Erlenmeyer flask was added 200mL of distilled water and the vegetal mass weighed 1.9g per 100 mL of water. Then, the water was added slowly with a magnetic stirrer to dissolve the mixture uniformly. Heating was done until the agar turned clear (approximately 20 minutes). One mL of glycerin was added and stirring continued for another 3 to 5 minutes. The flask was then removed from the heating source and covered with aluminum in autoclave for 15 minutes at 121°C.
Preparation of antimicrobial solutions
The isoniazid was used as reference substance for testing. Thus, it was used in various concentrations in order to prepare antimicrobial solutions for the experiments. Finally, 1mL of the solution was prepared, and added to the various concentration of 0.2μL up to 1.0μL of the testcompounds in ethanol.
The prepared concentrations were tested against the reference compound ethambutol. It was prepared by using 1mL of the solution along with 5 to 10μL preparations for each ester and thioester compounds. The last prepared solutions were used under different concentrations from 10 to 20μL.24
Preparation of petri plates
Inoculation and incubation
Preparation of the Ziehl-Neelsen stain
Procedure to realize this stain:
QSAR models
The QSAR studies were generated with the computational program Molecular Operating Environment (MOE) for 200 derivatives of INH. They were obtained from database bank at NIH.25–27 The QSAR modeling technique and its problems are described in the literature.28
QSAR study analysis
INH has been considered as a lead compound in the literatures.25–27 Each of the intermediate compounds from INH has an MIC value ≥ INH.15 In the analysis of the hydrazine derivatives of INH, the first mathematical equation is related to eight parameters as reported in Equation 1. On critical analysis, it was however discovered that isoniazid derivatives have problems with the incubation conditions and decay of derivatives would therefore have a consequence that would alter the microbiological potency measurements of the experiment.15 In this regard, our experiment would provide a useful insight.
The QSAR study’s result was an equation that related biological activity to molecules’ physicochemical properties. Using multiple linear regressions, a numerical relation (see equation below) between chemical structures with physicochemical properties and its biological activity was obtained. The final equation showed a linear correlation coefficient R2of 0.75. (R2=1, reflects a straight line, and represents a high linear correlation; R2<0.5 means there is a poor linear correlation).
With the values, n = 60; R2 = 0.75 the following was obtained:
pMIC = 5.41532 - (0.00416* Hydrophobic VdW surface area) - (2.65893* AM1 imino-carbonyl charges) + (0.16648* Kier- topological flexibility index)- (0.93573* Molecular shape) - (0.00751* Molecular volumen) + (0.23376* AM1 HOMO).
The model is barely logic in the chemical-microbiological interpretation. The analyzed parameters are contradictory in some cases. It should consider other possible explanations to help understand why the reported values of the 60 compounds from the QSAR studies do not correlate with the interpretation of its parameters calculatedand such explanations could be as follows:
|
QSAR models |
|
|
|
|
|
Descriptors Used |
1st |
2nd |
3rd |
|
|
X1 |
Octanol distribution coefficient/water Log P |
* |
||
|
X2 |
Hydrophobic VdW Surface area |
* |
* |
* |
|
X3 |
AM1 Imino-Carbonyl Charges |
* |
* |
* |
|
X4 |
Sum of bond orders |
* |
||
|
X5 |
Maximun electrotopological positive Variation |
* |
||
|
X6 |
Kier topological flexibility index |
* |
* |
* |
|
X7 |
Zagreb topological index M2 |
* |
||
|
X8 |
Molecular Acidity (pKa) |
* |
||
|
X9 |
Molecular form |
* |
* |
|
|
X10 |
Molecular volume |
* |
* |
|
|
X11 |
AM1 HOMO |
* |
* |
|
|
|
Number of descriptores |
8 |
6 |
6 |
Table 1 Descriptors X used in the three QSAR models
Equation 1: pMIC = -0.93551 + (0.33301* X1) - (0.89074* X2) - (0.41049* X3) - (0.48897* X4) + (0.33167* X5) + (0.27508* X6) + (0.11044* X7) + (0.15883* X8), with n=60; R2 = 0.596.
In the equation 1, two contradictions were observed for the polar - non polar area contributions. If the nonpolar character is increased in the molecule, the activity concentration (pMIC) is also expected to increase while the polar character should decrease. The nonpolarity can be quantified with X1: Log P and X2: Hydrophobic VdW surface area, with X3: AM1 imino-carbonyl charges (partial atomic charges by AM1 of “C” carbonyl and “O” atom), thus reflects polarity. TheX8: Molecular acidity, which correlates with Hammett sigma or a cationic charge (positivation) atomic partial charge. From the results obtained, it is observed that the nonpolar character shows a positive sign in X1 which is in contradiction to the negative sign in x2. Similarly, the positive sign of X8 which indicates deprotonization or dissociation into ions is a contradiction to the positive sign in X4.
Concerning the first contradiction
The coefficient of molecular descriptor X1: Log P was positive; showed that the biological activity increases, along with increase in nonpolarity. Nonetheless, (b) the b2 coefficient of molecular descriptor X2 is negative, the biological activity also increased in order to decrease the nonpolarity. Whereas an equation cannot exist with such contradictions; if a nonpolarity character increases, then all thecharacters with the same type of parameters should show increase.
The second contradiction was the following
The charges tend to become a value of zero for increasing the biological activity, this means that the charges should lose positive or negative charges, as it is shown with the descriptor X3. (b) If the acidity should increase, then consequently, the biological activity should increase. This is also a sort of “positivation” as shown on the descriptor X8. It is not possible to have two parameters that imply polarity, and yet both act inversely.
A second study used the following mathematical equation using six parameters which are reported in Equation 2.
Equation 2: pMIC = - 0.09070 - (0.28233* X2) - (0.17931* X3) - (0.13868* X6) - (0.45384* X9) - (0.27005* X10) + (0.06250* X11), with n = 60; R2 = 0.784.
In this model two contradictions were observed.
First contradiction
Second contradiction
A third mathematical model was created by Equation 3.
Equation 3: pMIC = 5.41532 - (0.00416* X2) - (2.65893* X3) + (0.16648* X6) - (0.93573* X9) - (0.00751* X10) + (0.23376* X11), with n = 60; R2 = 0.754.
Equation 3 of QSAR model number 3 study obtained two contradictions again in the polarity - non polarity area. In this equation, it manifested a first discrepancy: two contrary parameters cannot work in the same direction, as it is the case with hydrophobicity (X1) and polarity (X3). With regard to the second inconsistency, the charges with the quantum chemistry AM1 (X3) method correlates positively with the value of pMIC; nevertheless, the charges of imino-carbonyl (X2) correlates negatively. The two parameters under investigation with the same type of character have to act in the same direction. In this case, the biological activity constitutes the potential of antibiotic effect. The attraction between “C” carbonyl charges and “O” atoms of the parameter X2 greatly determines its activity for the trend of the metabolic breakdown of INH. Concerning the decrease in molecular volume, parameter X5, a maximum electro-topological positive variation could reflect a better reception through passive diffusion.
The linear relation in the molecules led to the particular analysis of the molecules and the way the values of reported pMIC were related. Studying the most usual procedures of Mtb incubation, it was found that it consists of an aqueous acidic medium as liquid phase, with glutamic acid as catalyst, high temperature, exposing during days.15 This allows degradation of hydrazones in INH and other products. Because MIC is reported, it is not necessarily the report about the activity of its initial molecule. In most of the cases it was degraded otherwise. Only one INH concentration was reported that is not identical to the initial concentration of the microbiological experiment and the other nonregistered products.15 However, in some other cases, it was already interpreted that the toxic molecules of hydrazine had a stronger antibacterial activity than INH.15 The reasons were as follows: there are substances in its composition that act while INH is liberated by the hydrolysis of these molecules. These molecules are capable of acting slowly in the mycobacterium. PABA was identified a bacterial nutrient like in these cases. The other reason was that when the molecule is hydrolyzed, and finally INH is obtained from the expected active concentrations of the initial molecule.15 Hence, its MIC is only apparent and the true active concentration becomes that of INH because the initial molecules (prodrugs) are converted into INH (drug). However, INH acts as the principal molecule at the end. Other possible reason for the variations in the reported values of MIC was that it is possible during the point of determination that some strains with high bacterial resistance were introduced by impure cultures or microbial ambient contamination.
The QSAR study of this study allowed us to observe a series of errors that affected the calculus of the models and the equations of the correlations. It has been reported [28] that:
Generating a fourth QSAR model with 57 derivatives of hydrazones.
A QSAR study with 57 hydrazone derivatives of INH was carried out (Figure 3). The molecules were all manually selected from NIAID (National Institute of Allergy and Infectious Diseases) at http://apps1.niaid.nih.gov/struct_search/ The flow analysis of a QSAR type study was as follows: In the first place MIC values were collected to represent the biological activities of the molecules and the physicochemical parameters for the derivatives were calculated. Then multiple linear regression (MLR) was applied to correlate the molecular properties (independent variables) with the biological end points (dependent variable, activities). Then the QSAR model equations were generated.28

Figure 3 The QSAR studies allowed us to interpret that INH derivatives are also precursors and their efficiency depends on conversion in INH or INA. None of the esters and thioesters of INA inhibited the growth of mycobacteria.30
The parameters were calculated for the QSAR study and the following final equation of MLR was obtained as follows:
MIC = -0.45* (Shape) - 0.28* (PEOE_VSA_HYD) - 0.27* (Vol) - 0.18* (q_R_AM1-Mul) + 0.14* (KierFlex) + 0.06* (AM1_HOMO)
The resulting coefficient of correlation was in the upper range of values (0.7 to 0.9) which represents a reliable QSAR model, namely R2=0.8.This means that this value can be accepted, because it is close to 1, the ideal point.
Analyses of the antibiograms
The esters were prepared by esterification of the isonicotinic acid with alcohol (1-butanol, 1-hexanol and 1-octanol) that was catalyzed by H2SO4. The confirmation of the ester formation was made by NMR of 1H where it showed the presence of the characterized signals of the isonicotinic acid’s aromatic ring and the corresponding signals of the alcohol residue. The structure was confirmed with the NMR data of the 13C and IR and with the literature data.29
Thioesters were prepared by transforming isonicotinic acid with isonicotyl chloride and by also reacting it with the corresponding thiol. The obtained thioesters were identified with its spectroscopy data (UV, IR and NMR) of 1H and 13C in similar like esters (Table 2).
|
Thioester |
UV (EtOH) λmax nm (log e) |
IR (CHCl3) cm-1 |
NMR-1 H dppm (J) |
NMR-13 C dppm |
|
S-butyl pyridine-4-carbothioate |
217 (4.02); 273 (3.86) |
3085, 2956, 2921, 1686, 1234, 1200, 973. |
8.76 (dd, 4.4, 1.6 Hz) (H-2); 7.73 (dd, 4.4, 1.6 Hz), 7.73 (dd, 4.4, 1.6 Hz), 8.76 (dd, 4.4, 1.6 Hz), 3.10 (t, 7.4 Hz), 1.68 (brquint, 7.4 Hz), 1.45 (br sext, 7.4 Hz), 0.95 (t, 7.4 Hz). |
149.7, 119.5, 142.2, 119.5, 149.7, 189.2, 29.1, 31.4, 27.2, 13.8. |
|
S-hexyl pyridine-4-carbothioate |
216 (4.01); 273 (3.85) |
3085, 2957, 2921, 1661, 1200, 1191, 991 |
8.76 (dd, 4.4, 1.6 Hz), 7.73 (dd, 4.4, 1.6 Hz), 7.73 (dd, 4.4, 1.6 Hz), 8.76 (dd, 4.4, 1.6 Hz), 3.08 (t, 7.4 Hz), 1.67 (brquint, 7.4 Hz), 1.2-1.5 m, 0.89 (br t, 6.5 Hz). |
149.3, 119.1, 141.8, 119.1, 149.3, 189.2, 28.6*, 29.0*, 28.3*, 31.0, 22.3.* May be interchanged |
|
S-octyl pyridine-4-carbothioate |
216 (4.06); 273 (3.90) |
3090, 2939, 2869, 1670, 1234, 1217, 956 |
8.75 (dd, 4.4, 1.7 Hz), 7.72 (dd, 4.4, 1.7 Hz), 7.72 (dd, 4.4, 1.7 Hz), 8.75 (dd, 4.4, 1.7 Hz), 3.07 (t, 7.0 Hz), 1.67 (brquint, 7.0 Hz), 1.2-1.5 m, 0.88 (br t, 6.6 Hz). |
149.4, 119.2, 141.9, 119.2, 149.4, 189.3, 28.8*, 29.0*, 28.9*, 29.1, 29.1, 31.6, 22.5, 14.1. |
Table 2 Clinical and biochemical variables of individuals with overweight-obesity
SD, standard deviation; BMI, body mass index; WC, waist circumference; AC, abdominal circumference; HC, hip circumference; RER, respiratory exchange ratio; HR, hear rate
The prepared compounds were proved against Mtb. The main observations of our experiment reflected that there was no inhibition of the Mycobacterium tuberculosis growth. Hence, it was concluded from the obtained result of the synthesized esters that the isonicotinic acid is not the active form of the isoniazid.30
Isoniazid (INH) is among the antibiotics of first line for the treatment of tuberculosis. INH is a prodrug activated by catalase-peroxidase (KatG) of Mycobacterium tuberculosis (Mtb). The active form is poorly characterized. There are two possible ways of INH activation. The active form interferes in the synthesis of my colic acids which are essential to mycobacterium. It hinders the enoil-reductase (InhA). Our study was intended to contribute to clarify the mechanism of action of the first line drug INH. Its derivatives were designed and synthesized to be tested. These compounds are ester derivatives of INA that should have antibiotic activity in the Mtb strains when they are activated by intracellular unspecific esterases. Furthermore, the corresponding thioesters were used as a negative control group in our study. On the one side, the inherent risk of ester building is the decrease in water solubility. On the other side, the esters are lipophilic and permeation of lipid compartments and barriers (cell walls etc) should be possible. So, a trade-off (not too long, not too short) for the ideal chain length of esters was necessary. Microbiological tests to evaluate the pharmacologic activity were carried out withthe compounds. Concerning the widely accepted mechanism, it has been proposed that KatG is responsible for the production of Isonicotinic acid’s (INA) radicals that associate with NAD. Secondly, another hypothesis postulated that intracellular accumulation of INA replaces the nicotinic acid in the NAD synthesis in order to produce iso NAD. The analysis of the antibacterial effects of the INA’s esters was negative. The interpretation of the negative results is as follows: either INA is most likely not the active form or the esters could not enter the bacterial cells, due to insufficient balance between water and lipid solubility. The drug resistance phenomenon cannot be ruled out, despite the positive susceptibility (the sensitivity pattern) of the Mtb reference strains, but mutations characterized in KatG or InhA could confer detrimental drug resistance, all of which would affect the results.
We are much indebted to Thomas (alias Tom) Scior, at the Pharmacy Department of Facultad de Ciencias Químicas, Universidad Autónoma de Puebla, Mexico, for directing the PhD work of JAJR, manuscript reading and language corrections, and assistance in all stages of the editorial procedure.
The authors declare there is no conflict of interests.
None.

©2016 Fadipe, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.