Journal of
eISSN: 2473-0831


Research Article Volume 10 Issue 6
1Department of Pharmacology /Toxicology, Faculty of Pharmacy, Niger Delta University, Bayelsa State, Nigeria
2Department of Biology, Faculty of Natural and Applied Sciences, Ignatius Ajuru University of Education, Rumuolumeni, Port Harcourt, Rivers State, Nigeria
3Department of Pharmacology, Faculty of Basic Clinical Sciences, University of Port Harcourt, Rivers State, Nigeria
Correspondence: Elias Adikwu, Department of Pharmacology /Toxicology, Faculty of Pharmacy, Niger Delta University, Bayelsa State, Nigeria
Received: November 30, 2021 | Published: December 29, 2021
Citation: Adikwu E, Ajeka IS, Ebong NO. Sulfadoxine/pyrimethamine/moxifloxacin as an antimalarial drug: A study in Plasmodium berghei-infected mice. J Anal Pharm Res. 2021;10(6):254-258. DOI: 10.15406/japlr.2021.10.00393
The use of triple antimalarial regimen may prevent Plasmodium parasites resistance. Moxifloxacin (M), a fluoroquinolone antibiotic has been associated with antiplasmodial activity. This study assessed the ability of M to increase the antiplasmodial activity of sulfadoxine/pyrimethamine (S/P) in mice infected with Plasmodium berghei. Adult Swiss albino mice (30-35g) of both sexes were randomly grouped and infected with Plasmodium berghei intraperitoneally. Curative, prophylactic and suppressive methods were followed and the mice were orally treated with S/P (21.4/10.7 mg/kg), M (6 mg/kg) and S/P/M, respectively. Chloroquine CQ (10mg/kg) served as the standard control. After treatment, blood samples were assessed for percentage parasitemia and hematological indices. The mice were observed for mortality expressed as mean survival time (MST). Liver samples were evaluated for changes in histology.
Results: The curative, prophylactic and suppressive tests, showed decreased percentage parasitemia in mice treated with S/P/M with significance observed at p<0.05 when compared to S/P or M. In the curative test, 70.0 %, 67. 5% and 90.1% parasitemia inhibitions were produced by S/P, M and S/P/M respectively, whereas CQ produced 87.5 % inhibition. In the curative, prophylactic and suppressive tests, S/P/M prolonged MST with significant difference observed at p<0.05 when compared to S/P or M. Treatment with S/P/M restored hematological indices characterized by significantly increased hemoglobin, packed cell volume, and red blood cells, and significantly decreased white blood cells at p<0.05 when compared to S/P or M. S/P/M eradicates liver Plasmodium parasite in treated mice. M increased the antiplasmodial activity of S/P. S/P/M may be used for malaria treatment.
Keywords: sulfadoxine/pyrimethamine, moxifloxacin, malaria, plasmodium, mice
Malaria is a severe public health challenge worldwide. It is a leading cause of morbidity and mortality in sub Saharan African countries where its burden is high. In 2018, sub Saharan Africa accounted for 94% of world malaria deaths, of which 67% were children under five.1,2 Globally, artemisinin-based combination therapies (ACTs) used for the treatment of malaria have greatly contributed to the reduction of malaria burden.3 However, there is an emergence and spread of Plasmodium resistance to ACTs,4 which has led to decreased therapeutic efficacy.4,5 In the absence of new compounds, a fast approach to address Plasmodium resistance may involve the use of triple ACTs where artemisinins are combined with two existing partner drugs.6 Such triple-drug combinations are now standard for the treatment of tuberculosis and human immunodeficiency virus infections.7,8 Compared to ACTs, this could sustain the efficacy of drugs over longer periods, even in the context of artemisinin resistance, wherein partner drugs are exposed to a higher parasite biomass, and are thus more prone to the development of resistance.6
Sulfadoxine/pyrimethamine (S/P) is used for the treatment of malaria in developing countries9 and is a cornerstone for malaria chemoprophylaxis. Sulfadoxine inhibits dihydropteroate synthase, a key enzyme in the biosynthesis of folate.10 Pyrimethamine inhibits dihydrofolate reductase, a key enzyme in the redox cycle for the production of tetrahydrofolate.11,12 S/P exerts synergism against malaria parasites. It is used in combination with artesunate in some African countries for the treatment of malaria.13 The use of S/P remains an effective strategy for the intermittent preventive treatment of malaria in pregnancy where it decreases placental parasitaemia and improves birth outcomes.14 However, Plasmodium parasites resistance especially Plasmodium falciparum to S/P threatens its antimalarial effectiveness in intermittent preventive treatment during pregnancy and its use for the treatment of malaria in sub-Saharan Africa.15 Plasmodium parasites resistance to S/P is attributed to mutations in parasite’s dihydrofolate reductase and dihydropteroate synthase genes.16
Moxifloxacin (M) is a fourth-generation fluoroquinolone antibacterial agent with a broad spectrum of activity. It has enhanced activity against gram-positive bacteria in comparison to ciprofloxacin.17 It binds to DNA gyrase (topoisomerase II) and topoisomerase IV thereby preventing DNA replication and repair leading to bacterial death.18 In addition to its antibacterial activity, M and other fluoroquinolones have promising antimalarial activity. In-vitro studies showed the antiplasmodial activity of M against chloroquine-sensitive and chloroquine resistant strains of Plasmodium falciparum.19 Fluoroquinolones target several stages of malaria parasite life cycle, including the erythrocytic, hepatic and gametocytes stages.20 The antimalarial activity of fluoroquinolones is possibly by targeting the gyrase of Plasmodium an enzyme involved in apicoplast DNA replication.21,22 Studies suggested the combination of fluoroquinolones with artemisinin derivatives and other antimalarial drugs for improved antimalarial activity.20 Hence, this study, assessed the antiplasmodial effect of sulfadoxine/pyrimethamine/moxifloxacin on Plasmodium berghei-infected mice.
Experimental animals
Adult Swiss albino mice (30-35g) of both sexes used were purchased from the animal facility of the Department of Pharmacology, Faculty of Basic Clinical Sciences, University of Port Harcourt, Rivers State, Nigeria. The mice were housed in standard cages and provided with standard pellet diet and water ad libitum. The mice were acclimated for 2 weeks before the study commenced and were maintained under standard laboratory conditions in accordance with the guide for the care and use of Laboratory animals 8th Edition.23
Plasmodium parasite and inoculation
Chloroquine (CQ) sensitivePlasmodium berghei (P. berghei) (NK65) was acquired from the Nigerian Institute of Medical Research, Yaba, Lagos State. The parasites were supplied in donor mice and kept alive by continuous intraperitoneal (ip) inoculation in mice after every four days. The inoculum was prepared by collecting blood infected with P. berghei from donor mice into tubes containing 1 %( w/v) heparin. The parasitized blood was diluted with isotonic saline to attain roughly 0.2 ml of the blood solution containing approximately 1×107 parasitized erythrocytes.
Drugs
Sulfadoxine/Pyrimethamine (S/P) manufactured by Swiss Pharma Nigeria Limited, moxifloxacin (M) manufactured by Torrent Pharm Limited, India and Chloroquine (CQ) manufactured by Evans Pharm, Nigeria were used. The following doses were used: S/P (21.4/10.7 mg/kg),24 M (6 mg/kg),25 and CQ (10mg/kg).26
Antiplasmodial test
Curative test
The curative test was carried out as described by Ryley and Peters.27 Twenty-five adult Swiss albino mice inoculated with P. berghei (1×107) (ip) and randomized into 5 groups (A1-A5) of 5 mice each were used. The mice were orally treated as follows; group A3 was treated with a dose of S/P (21.4/10.7 mg/kg), group A4 was treated daily with M (6 mg/kg) for 4 days, and group A5 was treated with S/P/M (a dose of S/P and daily dose of M for 4 days). Groups A1 and A2 the negative and positive controls were treated daily with normal saline (0.2mL) and CQ (10mg/kg), respectively for 4 day. On the 5th day, blood samples were collected from the tail of the mice on slides and thin blood film were produced. The slides were stained with 10% Giemsa stain at pH 7.2, rinsed with distilled water and air dried at room temperature. The slides were assessed for percentage parasitemia and percentage inhibitions using the formula below.
Suppressive test
The suppressive test was evaluated as explained by Knight and Peters.28 Twenty-five adult Swiss albino mice, grouped randomly into 5 (BI-B5) of 5 mice each were inoculated with P. berghei (1×107) (ip). The mice were allowed for 2 hr before treatment. Group BI (negative control) and group B2 (positive control) were treated daily with normal saline (0.2mL) and CQ (10mg/kg), respectively for 4 days. Group B3 was treated with a dose of S/P (21.4/10.7 mg/kg), group B4 was treated daily with M (6 mg/kg) for 4 days, and group B5 was treated with S/P/M ( a dose of S/P and daily dose of M for 4 days). After, drug treatment, on day 5, blood samples were collected from the tail of the mice and thin film prepared on slides. After fixation with alcohol and staining with 10% Giemsa stain at pH 7.2, the slides were rinsed with distilled water and air dried at room temperature. The slides were assessed for percentage parasitemia and percentage inhibitions using the formula above.
Prophylactic test
The prophylactic test was assessed using the method described by Peters.29 Twenty-five adult Swiss albino were randomly grouped in to 5 (C1-C5) of 5 mice/group. Group C1 (negative control) and group C 2 (positive control) were orally treated daily for 4 days with normal saline (0.2mL) and CQ (10mg/kg), respectively. Group C3 was treated with a dose of S/P (21.4/10.7 mg/kg), group C4 was treated daily with M (6 mg/kg) for 4 days. Group C5 was treated with S/P/M (a dose of S/P and daily dose of M for 4 days). On the 5th day, the mice were inoculated with P. berghei (1 × 107) (ip) and allowed for 3 days. Thereafter, tail blood samples were collected on slides. Slides were fixed with alcohol, stained with 10% Giemsa at pH 7.2 and rinsed with distilled water. Slides were air dried at room temperature and assessed for percentage parasitemia and percentage inhibitions using the formula above.
Monitoring of mean survival time
Mortality was observed in the controls and treated groups. The number of days from the time of inoculation with parasite to death was recorded for all the mice per group. The mean survival time (MST) for each group was calculated with the formula below:
Hematological analysis
Blood samples obtained from the mice in the curative test were evaluated for red blood cells (RBCs), white blood cells (WBCs), packed cell volume (PCV) and hemoglobin (Hb) with the aid of an auto analyzer.
Histology of the liver
Liver tissues from the control and treated groups in the curative test were dissected, immersed in 10% formalin saline for 24hr and dehydrated in graded alcohol concentrations. Liver tissues were prepared and embedded in paraffin block. Liver tissues were sectioned (3µm each) and stained with Haematoxylin and Eosin on slides and examined with the aid of a light microscope for histological changes.
Statistical analysis
Data (n=5/group) were presented as mean ± S.E.M. Data were analyzed using one way analysis of variance (ANOVA) with the aid of Graph Pad Prism followed by Tukey’s multiple comparison tests. The result was considered statistically significant at p<0.05.
Curative antiplasmodial effect
Treatment with S/P/M decreased percentage parasitemia with significant difference observed at p<0.05 when compared to S/P or M. M, S/P and S/P/M produced parasitemia inhibitions of 65.70%, 70.51%, and 90.12%, respectively while CQ produced 80.52% parasitemia inhibition (Table 1). Treatment with S/P/M significantly prolonged MST with difference observed at p<0.05 when compared to S/P or M (Table 1).
|
Treatment |
% Parasitemia |
% Inhibition |
MST(Days) |
|
NC |
32.31±3.72 |
0.00 |
9.12±0.34 |
|
CQ |
4.03±0.31a |
80.52 |
29.0±2.00a |
|
S/P |
9.53±0.92b |
70.51 |
17.7±1.55b |
|
M |
11.08±0.35c |
65.70 |
23.6±3.67b |
|
S/P/M |
3.19±0.11d |
90.12 |
33.2±3.50a |
Table 1 Curative effect of sulfadoxine/pyrimethamine/moxifloxacin on Plasmodium berghei-infected mice
NC: Negative control, CQ: Chloroquine, S/P: Sulfadoxine/pyrimethamine, M: Moxifloxacin. Values with different superscripts down the column differ significantly at p<0.05 (ANOVA)
Suppressive antiplasmodial effect
S/P/M significantly (p<0.05) decreased percentage parasitemia when compared to S/P or M. The parasitemia inhibitions produced by M, S/P and S/P/M represent 68.61%, 78.53%, and 95.88%, respectively whereas CQ produced 85.00 % parasitemia inhibition (Table 2). MST was prolonged by S/P/M with significant difference observed at p<0.05 when compared to S/P or M (Table 2).
|
Treatment |
% Parasitemia |
% Inhibition |
MST(Days) |
|
NC |
24.98±3.24 |
0.00 |
9.27±0.54 |
|
CQ |
2.25±0.14a |
85.00 |
31.36±3.10a |
|
S/P |
5.36±0.57b |
78.53 |
20.18±2.61b |
|
M |
7.84±0.57c |
68.61 |
25.34±3.55c |
|
S/P/M |
1.03±0.09d |
95.88 |
37.08±4.21d |
Table 2 Suppressive effect of sulfadoxine/pyrimethamine/moxifloxacin on Plasmodium berghei-infectedmice
NC: Negative control, CQ: Chloroquine, S/P: Sulfadoxine/pyrimethamine, M: Moxifloxacin. Values with different superscripts down the column differ significantly at p<0.05 (ANOVA)
Prophylactic antiplasmodial effect
Treatment with S/P/M decreased percentage parasitemia with significant difference observed at p<0.05 when compared to S/P or M (Table 3). The parasitemia inhibitions which represent 70.54%, 80.27% and 98.79% were produced by M, S/P and S/P/M, respectively while 90.00% parasitemia inhibition was produced by CQ (Table 3). S/P/M prolonged MST significantly with difference observed at p<0.05 when compared to S/P or M (Table 3).
|
Treatment |
% Parasitemia |
% Inhibition |
MST(Days) |
|
NC |
18.45±3.33 |
0.00 |
9.57±0.16 |
|
CQ |
1.29±0.61a |
90.00 |
34.17±3.61a |
|
S/P |
3.64±0.56b |
80.27 |
23.62±3.36b |
|
M |
5.44±0.64c |
70.54 |
28.86±3.57c |
|
S/P/M |
0.22±0.01d |
98.79 |
40.71±4.33d |
Table 3 Prophylactic effect of sulfadoxine/pyrimethamine/moxifloxacin on Plasmodium berghei-infectedmice
NC: Negative control, CQ: Chloroquine, S/P: Sulfadoxine/pyrimethamine, M: Moxifloxacin. Values with different superscripts down the column differ significantly at p<0.05 (ANOVA)
Effect on hematological indices
Reduced RBCs, PCV and Hb with increased WBCs occurred in P. berghei-infected mice (Table 4). But treatment with S/P/M significantly increased RBCs, PCV and Hb and significantly decreased WBCs with difference observed at p<0.05 when compared to S/P or M (Table 4).
|
Treatment |
RBCs (x106) |
WBCs (cells/L) |
PCV (%) |
Hb (g/dL) |
|
C |
7.88±0.15 |
6.98±0.68 |
60.09±6.47 |
16.70±1.34 |
|
NC |
2.51±0.72a |
18.61±2.33a |
20.79±3.22a |
6.33±0.17a |
|
CQ |
6.00±0.41b |
8.81±0.61b |
49.55±5.41b |
13.06±1.06b |
|
S/P |
4.21±0.33c |
11.36±1.40c |
38.31±3.66c |
10.01±0.27c |
|
M |
3.14±0.28d |
13.00±1.33d |
30.74±2.34e |
10.00±0.59c |
|
S/P/M |
7.62±0.67e |
6.67±0.45e |
57.73±5.61d |
16.07±1.66d |
Table 4 Effect of sulfadoxine/pyrimethamine/moxifloxacin on hematological parameters of Plasmodium berghei-infected mice
C: Normal control, NC: Negative control, CQ: Chloroquine, S/P: Sulfadoxine/pyrimethamine, M: Moxifloxacin. RBCs: Red blood cells, WBCs: White blood cells, PCV: Packed cell volume, Hb: Hemoglobin, Values with different superscripts down the column differ significantly at p<0.05 (ANOVA)
Effect on liver histology
The liver of normal control showed normal hepatocytes, Sinusoids, and central vein (Figure A), whereas the liver of the negative control showed steatosis, central vein congestion, inflammatory cells and merozoites ((Figure B)). The liver of CQ-treated mice showed normal hepatocytes, Sinusoids, and central vein (Figure C). The liver of M-treated mice showed normal hepatocytes, Sinusoids, and central vein congestion while. The liver of S/P-treated mice showed normal hepatocytes, central vein, and Sinusoids (Figures D and E). The liver of S/P/M-treated mice showed normal hepatocytes, Sinusoids, and normal central vein (Figure F).
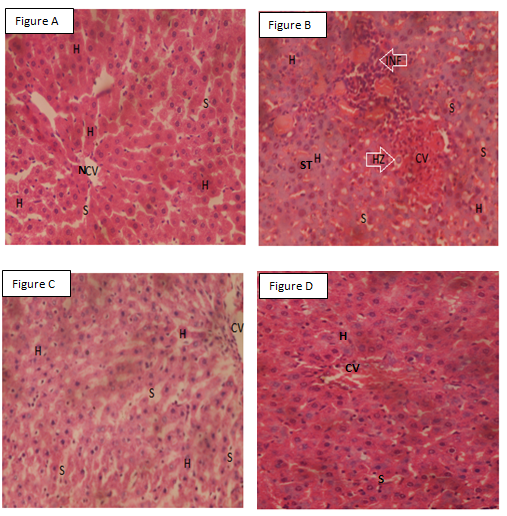
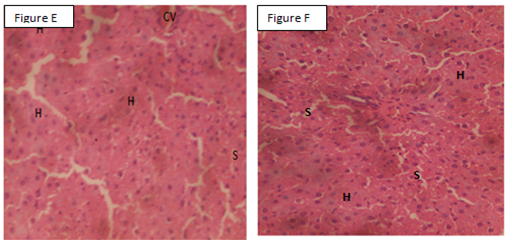
Figure A: Liver of control mice. Figure B: Liver of parasitized mice, Figure C: Liver of parasitized mice treated with chloroquine. Figure D: Liver of parasitized mice treated with moxifloxacin. Figure E: Liver of parasitized mice treated with sulfadoxine/pyrimethamine. F: Liver of parasitized mice treated with sulfadoxine/pyrimethamine/moxifloxacin. INF: Inflammatory cells, NCV: Normal central vein, HZ: Merozoites, S: Sinusoids, Normal hepatocytes, CV: Congested central vein, ST: Steatosis X 400 H&E.
Malaria still imposes a significant health and economic burden in large parts of the world, particularly in sub-Saharan Africa and Southeast Asia, where at least 200 million infections and over 600,000 deaths are registered annually.30 Artemisinin-based combination therapies (ACTs) are used worldwide as the first-line treatment for malaria.31 Despite the use of ACTs, the treatment of malaria is a big challenge due to the increasing cases of Plasmodium resistance to the majority of currently available antimalarial drugs including ACTs.32 The development of triple artemisinin-based combination therapy could contribute to mitigating the risks of Plasmodium resistance.33 This study assessed the antiplasmodal effect of S/P/M on P. berghei infected mice. A mouse model was used for this study, because it permits investigation into disease progression, which seems difficult in humans. It also allows the assessments of organs such as the liver, which parasite can hibernate.34 In-vivo antimalarial study is the foremost broadly utilized method to assess the antimalarial activity of a potential antimalarial drug moiety.
A four-day suppressive test is one of the most extensively used test to study the antiplasmodial activity of new entities.35 The curative test is used to study the antiplasmodial activity of a test compound in an established infection.36 In this study, S/P/M produced notable parasitemia inhibitions in the curative, suppressive and prophylactic tests compared to S/P and M alone. MST is an important parameter for the antiplasmodial assessments of potential drug moieties. The prolongation of MST by a potential drug moiety greater than the negative control gives credence to its possible antiplasmodial activity.37 The current study observed that S/P/M prolonged MST best in comparison to individual doses of S/P and M. Severe malaria-related anemia is a major cause of morbidity and mortality in endemic areas.38 Malaria-related anemia is multifactorial with overlapping features, such as lysis of RBCs, splenic sequestration of RBCs, dyserythropoiesis and bone marrow suppression.33 The assessments of hematologic markers including RBCs, Hb, PCV and WBCs provide enormous information on malaria-related anemia.39,40 In this study, altered hematologic markers marked by decreased RBCs, Hb, PCV and increased WBCs were noted in P. berghei-infected mice. These are signs of anemia which support earlier reports.32 But S/P/M produced notable reduction in in anemia, marked by restored levels of RBCs, Hb, PCV and WBCs best than S/P and M alone.
This study assessed the ability of S/P/M to eradicate the liver stage of malaria infection to buttress its antiplasmodial activity. Malaria parasites undergo replication and population expansions within the vertebrate host. Host infection initiates with sporozoite invasion of the liver, which is followed by a dramatic parasite amplification. During liver stage, parasites grow and replicate within hepatocytes.41 The invasion of the hepatocytes by parasites may lead to perturbations such as steatosis, inflammation, and central vein congestion,36 which are consistent with the findings in the current study in P. berghei-infected mice. Interestingly, S/P/M eradicated liver stage of P. berghei infection best than S/P and M alone. The observation in this study may be due to the augmentation of the antiplasmodial activity of S/P by M. S/P act synergistically to inhibit two steps in folate synthesis pathway. S inhibits dihydropteroate synthetase enzyme while P inhibits dihydrofolate reductase enzyme.42 The mechanisms for the antiplasmodial action of M are not well define, but M might have inhibited gyrase of Plasmodium an enzyme involved in apicoplast DNA replication.21,22
M augmented the curative, suppressive and prophylactic antiplasmodial activities of S/P. This study suggests the clinical use of S/P/M for the treatment of malaria.
The authors kindly appreciate the technical assistance offered by Confidence Ogechi Nworgu of the Department of Biology, Faculty of Natural and Applied Sciences, Ignatius Ajuru University of Education, Rivers State, Nigeria.
None.
None.

©2021 Adikwu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.