Journal of
eISSN: 2473-0831


Research Article Volume 8 Issue 2
The Laboratory for Research on Bioactive Products and the Valorization of Biomass, Ecole Normale Supérieure, Algeria
Correspondence: Malki Fatiha, The Laboratory for Research on Bioactive Products and the Valorization of Biomass, Ecole Normale Supérieure, 16308, BP 92, Vieux-Kouba str., Algiers, Algeria
Received: June 30, 2019 | Published: March 18, 2019
Citation: Fatiha M, Abdelkader T. Study of antioxidant activity of pyrimidinium betaines by DPPH radical scavenging method. J Anal Pharm Res. 2019;8(2):33?36. DOI: 10.15406/japlr.2019.08.00308
The aim of this investigation was the study of the effect of some kinetic factors on the antioxidant activity of some synthesized pyrimidinium betaines, using 2, 2-diphenyl-1-picrylhydrazy (DPPH) free radical scavenging method. These betaines are bicyclics 1, 2 and monocyclic 3. We investigated the effect of concentration and reaction time on the antioxidant activity of these compounds and some standard antioxidants. All tested compounds were found to be active as DPPH scavengers but concentrations and reaction times influenced deeply the evaluation. At high concentrations, compound 3 is the best antioxidant compared to all other betaines. Reaction time is one of the most important criteria in this reaction. In comparison with standard antioxidants as ascorbic acid and the synthetic antioxidants butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA), the data shows that, depending on the reaction time used, a different activity of the compounds is obtained: standard antioxidants react immediately with DPPH, while pyrimidinium betaines require longer reaction times and higher concentrations.
Keywords: antioxidant activity, concentration effect, DPPH, pyrimidinium betaine, radical scavenging, reaction time effect
Free radicals cause various human diseases.1 Antioxidants are substances that eliminate free radicals and serve as a defensive factor against their effects in the body. Antioxidants play an important role in the prevention of diseases induced by free radicals2 and systematically reduce their risk by reducing their concentrations.3 They act by inhibiting the initiation and propagation steps, resulting in the end of the reaction and delaying the oxidation process.4 In biological systems, antioxidants have multiple functions, eg it defends against oxidative damage by participating in the main cell signaling pathways and by preventing cell damage caused by the action of reactive oxygen species (ROS). Therefore, antioxidant consumption and the addition of antioxidant in food materials protect the body as well as food from deterioration of oxidation.5 Numerous methods to evaluate the antioxidant activity of specific compounds have been described, but the most widely documented relates to the 2, 2-diphenyl-1-picrylhydrazyl radical (DPPH).6-9 DPPH is stable free radical that accepts an electron or hydrogen to become a stable diamagnetic molecule. In the DPPH radical-scavenging assay, antioxidants react with DPPH, and convert it to yellow- colored diphenylpicrylhydrazine. The color fading extent proves indirectly the radical-scavenging capacity of the antioxidant.6 The reduction of DPPH radical by antioxidants is evaluated by the decrease in absorbance at 517 nm. The decrease in absorbance of DPPH radical caused by antioxidants is due to the reaction between antioxidant molecules and radical progress which results in the scavenging of the radical by hydrogen donation.10 The DPPH tests provided in the literature are based on the same principle as described by Brand- Williams et al.,8 but the analytical protocols differ in several parameters. Evaluation of the methods and modifications for determination of the radical scavenging activity by DPPH shows that the main factors influenced the reproducibility are the solvent, duration of the reaction, sample to reagent ratio and the wave length for absorbance measurement of the decolouration of the reaction mixture.11 Therefore, it is obvious that these factors are of significance for the radical scavenging activity of the tested compounds.
Pyrimidinium betaines constitute a class of mesoionic compounds12 which have raised much interest as biologically active compounds.13 Pyrimidinium betaines remain an important class of bioactive heterocyclics due to the presence of the pyrimidine ring in their structure. The literatures indicated that compounds having pyrimidine nucleus have a broad range of biological. As a result of remarkable pharmacological activity of pyrimidine derivatives, intensive has been focused on these activities, thus constituting an integral part of pharmaceutical industry.14
In previous studies15,16 we have tested experimentally some synthesized pyrimidinium betaines through their ability to quench the synthetic 2,2- diphenyl-1-picrylhydrazyl (DPPH) radical. We have demonstrated in various in vitro assays16 that these compounds have antioxidative properties. In the present paper, the study of the reaction conditions for the determination of the activity of free radical scavenging by DPPH method was carried out. We investigated the effect of concentration and reaction time on the antioxidant activity of three synthesized pyrimidinium betaines and standard antioxidants, using 2,2-diphenyl-1-picrylhydrazy (DPPH) free radical scavenging. These betaines are, bicyclics 1, 2 and monocyclic 3 (Figure 1).
Material
Chemicals and all solvents were of analytical grade and were purchased from Sigma-Aldrich, Merck, Prolabo and Biochem. In this study, pyrimidinium betaines 1-3 dissolved in ethanol were tested at concentrations 0-500µg/mL. Butylated hydroxyanisole (BHA), Butylated hydroxy toluene (BHT) and ascorbic acid (vitamin C) used as control standard antioxidants dissolved in ethanol were tested at concentrations 0-15µg/mL.
Methods
Synthesis of the compounds: The compounds including bicyclic pyrimidinium betaines: 1, 2 and monocyclic 3 were synthesized from amine derivatives and malonic esters by the reported procedures17-20 and their structures were determined by spectroscopic methods. These molecules have a common structure; they are mesoionic compounds. Their chemical structures are represented in Figure 1. Details of the synthesis and structural characterization of the compounds 1-3 can be found in Malki et al.19-21
DPPH radical scavenging assay: DPPH has been widely used for measurement of free radical scavenging ability of antioxidants.22 This method is based on the reduction of an alcoholic DPPH solution in the presence of a hydrogen-donating antioxidant.23 Hydrogen atom or electron-donation ability of the corresponding compounds were measured spectrophotometrically from the bleaching of the purple-colored methanol solution of 2,2-diphenyl-1-picrylhydrazyl (DPPH).24 In this study, antioxidant activity of tested compounds was measured using the stable radical 2, 2- diphenyl-1-picrylhydraziyl (DPPH). The free radical scavenging capacity of betaines was determined using the DPPH according to the method of Blois with some modifications.6 A solution of DPPH in methanol (0.004%) was prepared and 1mL of this solution was mixed with 1mL of varying concentrations of betaines solution in ethanol. The reaction mixture was vortexed thoroughly and left in the dark at room temperature. The absorbance of the mixture was spectrophotometrically measured at λmax=517nm and compared to the standard antioxidants (BHT, BHA and ascorbic acid (vitamin C). DPPH radical scavenging activity (% RSA) of compounds was calculated from the absorbance at the start (0) and after some reaction time (T) according to the equation (1).
Where ABS is the absorbance of blank sample (DPPH) solution without the compound to be tested and ATS is the absorbance of tested sample.
Determination of radical scavenging activity by DPPH method
DPPH has been used extensively as a free radical to evaluate reducing substances25 and a reagent for investigating the free radical scavenging activities of compounds.26 As the electrons become paired off, the solution color faded stoichiometrically depending on the number of electron taken up.6 Hence, this assay provides an insight into the reactivity of the tested samples towards a stable free radical.27
In this study, the antioxidant scavenging activity of the tested compounds was studied by in vitro determination of DPPH scavenging capacity.15,16 and the effects of the concentration and the reaction time on their antioxidant activity were examined. Ascorbic acid, BHA and BHT were used as reference compounds.
Effect of the concentration
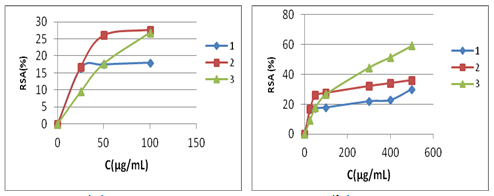
The results of DPPH radical scavenging rate (%) of studied betaines 1-3 at various concentrations at T=180min, are illustrated in Figure 2 &Figure 3.
a b
a) %RSA, C=100 mg/mL b) %RSA, Cmax= 500mg/mL
Figure 2 Radical scavenging activity of tested betaines 1-3.
From these figures, the DPPH radical scavenging activity towards pyrimidinium betaines is clearly demonstrated. As it can be seen, all tested betaines are active towards free radicals. The DPPH drop is concentration-dependent. The higher percentage of DPPH radical scavenging rate correlates with the higher concentrations.28 The decrease in the UV-visible absorbance of the DPPH radical engendered by tested betaines was due to the radical scavenging by electron donation.15 Results obtained in this study indicate different DPPH radical scavenging rate of tested compounds:
At lower concentrations (0- 25μg/mL) (Figure 2a & Figure 3a) the results show that, both bicyclic betaines 1and 2 exhibit the similar antioxidant activity that is higher than of monocyclic betaine 3. At 50μg/mL, bicyclic betaine 1 and monocyclic 3 have the similar activity (17,73%, and 18.01%) respectively, and at this concentration bicyclic betaine 2 is a stronger antioxidant (26, 04%) than previous betaines.
At 100μg/mL bicyclic betaine 2 has similar activity as monocyclic 3 (27, 67%, 26, 82%) respectively. At higher concentrations (200-500μg/mL) (Figure 2b& Figure 3b), monocyclic betaine 3 has the higher radical scavenging activity than both bicyclic betaines 1and 2, and reaches the highest antioxidant activity at 500μg/mL (59,20%) of DPPH radical scavenging rate, while bicyclics betaines 1and 2 only 30,19% and 36,18% respectively. The antioxidant activity of pyrimidinium betaines can be attributed to the conjugated systems involving nitrogen atoms which are known to stabilize free radicals.29 This conjugated systems are more extensive in monocyclic betaine 3 in comparison with the bicyclics 1 and 2. In 3 the radical formed is strongly stabilized by resonance through phenyl and carbonyl groups.16
On the basis of results obtained for all tested betaines, we concluded that at a low concentrations, bicyclic betaines show the highest DPPH radical scavenging rate, whereas at high concentrations monocyclic betaines show the highest DPPH radical scavenging rate. The radical scavenging activity (%RSA) of standard antioxidants (BHA, BHT and vitamin C) at concentrations 0-15μg/mL (T= 180 min) are shown in Figure 4.
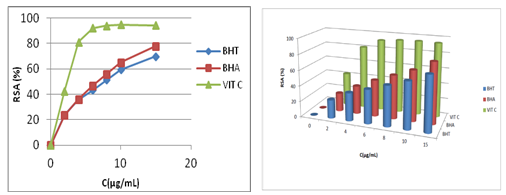
a b
Figure 4a) DPPH radical scavenging activity (%RSA) of BHA, BHT and vitamin C at concentrations 0-15µg/mL b) antioxidant profile of BHA, BHT, vitamin C at concentrations 0-15µg/mL).
The results indicate a significant decrease of DPPH radical due to the scavenging ability of tested standard antioxidants, and vitamin C is the most effective antioxidant. With regard to the molecular structures, the significant scavenging power of standards antioxidants was mainly due to the presence of hydroxyl groups in their structures on the phenyl ring which can donate hydrogen atom.30
Compared to the standard antioxidants (BHA, BHT and vitamin C), the tested betaines 1-3 show the lowest radical scavenging activity. Indeed, at lower concentrations (0- 15μg/mL) standard antioxidants (BHA, BHT and vitamin C) exhibit the highest antioxidant activity (70-94%) and betaines 1-3 only 5-10%. The low reactivity of pyrimidinium betaines towards DPPH radical can be attributed to the presence of the effects of steric hindrance and the nature of the substituents affixed on the hydrophilic head of betaine.16
We can conclude that scavenging activity differs between tested compounds. Since standard antioxidants increase the radical scavenging properties at a lower concentrations compared to the tested betaines.
Effect of the reaction time
Reaction time is one of the most important in the reaction,31 The DPPH radical scavenging activity determinations reported in literature are based on the DPPH absorbance decrease very often measured at different reaction times: 30 min in Blois method,6 shorter times (20, 10 and/or 5 min) from other authors.19-21 In this study, we followed kinetics’ reaction of betaines 1-3, keeping constant their concentrations. We studied the activity of the compounds’ concentrations (300 µg/mL) during 60 min and 180 min. Figure 5 & Figure 6 present the DPPH absorbance decrease as a function of time, in the presence of compounds 1-3 presented as (%RSA), according to the equation (1). The data show that, depending of the reaction time used, a different activity of the compounds is obtained:
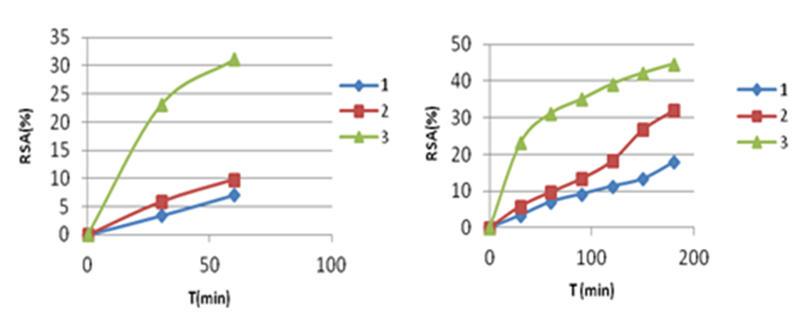
a b
a) %RSA, Tmax=180 min, b) %RSA, T=60 min
Figure 5 Radical scavenging activity of tested betaines 1-3 as a function of time (C=.300µg/mL).
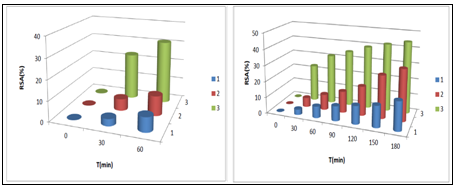
a b
a)%RSA, Tmax=180 min, b) %RSA, Tmax=60 min
Figure 6 Antioxidant profile of tested betaines 1-3 as a function of time (C=.300µg/mL).
In the time interval 0-60 min: we observed that bicyclic betaines 1 and 2 have almost the same activities, whereas monocyclic betaine 3 is more active compared to 1 and 2 (Figure 5a & Figure 6a).
At maximal reaction time (Tmax=180 min: monocyclic betaine 3 reaches the highest antioxidant activity (44.57 %), of DPPH radical scavenging rate which is close to that of bicyclic betaine 2 (32.02%), while bicyclic betaine 1 showed the weakest activity (17.85%) (Figure 5b & Figure 6b). We concluded that the reaction time profoundly influenced the antioxidant activity of pyrimidinium betaines and that compound 3 is the best antioxidant compared to all other betaines.
The presented study indicates the antioxidant potential of synthesized pyrimidinium betaines. The results demonstrated that at low concentrations bicyclic betaines 1 and 2 exhibit the highest DPPH radical scavenging whereas at high concentrations monocyclic betaine 3 shows the highest level radical scavenging properties. Among all tested betaines, compound 3 demonstrated strongest activity in all reaction times applied.
We can conclude that the time reaction and the concentration of the tested compounds are significant for their radical scavenging activity. Therefore, we can obtain much more information on the activity of the compounds by checking their reaction time and their concentrations. The results of this study will be useful for understanding and designing new experiences to evaluate the antiradical properties of chemically pure plant extract.
None.
The author declares that there is no conflicts of interest.

©2019 Fatiha, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.