
Research Article Volume 7 Issue 5
Stability constants of cerium(IV) complexes with 8-hydroxyquinoline and 8-hydroxy-7-iodoquinoline-5-sulfonic acid
Olga O Voskresenskaya,
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Nina A Skorik
Correspondence: Olga Voskresenskaya, Laboratory of Information Technologies, Joint Institute for Nuclear Research, 6 Joliot Curie, 141980 Dubna Moscow Region, Russia, Tel +7 916 373 8347
Received: August 06, 2018 | Published: September 4, 2018
Citation: Voskresenskay OO, Skorik NA. Stability constants of cerium(IV) complexes with 8-hydroxyquinoline and 8-hydroxy-7-iodoquinoline-5-sulfonic acid. J Anal Pharm Res. 2018;7(5):517?520. DOI: 10.15406/japlr.2018.07.00277
Download PDF
Abstract
Thermodynamic stability of cerium(IV) complexes formed in the initial stage of oxidation of 8-hydroxyquinoline and 8-hydroxy-7-iodoquinoline-5-sulfonic acid by cerium(IV) sulfate were studied spectrophotometrically and pH-potentiometrically with the aid of differential kinetic methods at an ionic strength I = 2 mol/L within the pH range of 0.5–2.5 in a sulfuric acid medium and at temperatures of –289.15 K. Composition of these complexes, the form of organic ligand existence therein, and their stability constants were determined.
Keywords: 8-hydroxyquinoline, 8-hydroxy-7-iodoquinoline-5-sulfonic acid, cerium, coordination compounds, stability constants
Introduction
8-hydroxyquinoline, its derivatives and metal-ion complexes exhibit multifunctional properties, including antioxidant, antineurodegenerative, anticancer, anti-inflammatory and antidiabetic activities. The interest in 8-hydroxyquinolines and their metal complexes has grown in the last two decades exponentially as they are privileged structures for the design of new drug candidates that exert a host of biological effects on various targets.1,2
The derivatives of 8-hydroxyquinoline and their compounds with rare earth elements (REE) are widely used in analytical chemistry. The luminescent properties of REE complexes with 8-hydroxyquinoline and its derivatives are employed in luminescent analysis, technologies for creating materials with photo- and electroluminescent properties for optoelectronics, photonics, chemo- and biosensorics.3,4 8-hydroxyquinoline is used in the extraction and spectrophotometric determination of cerium(IV), and cerium(IV) is employed in the oxydimetric determination of 8-hydroxyquinoline.5
However, there are no data on the thermodynamic stability of cerium(IV) complexes with 8-hydroxyquinoline (HOxiN) and 8-hydroxy-7-iodoquinoline-5-sulfonic acid (H2 Fer) in the literature. In this paper, the kinetic analogues of the thermodynamic methods for investigating the formation of variable-valence metal complexes are applied to the study of the stability of cerium(IV) complexes6,7 formed in the
systems with
and
,
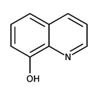

HOxN
Materials and procedure
Reagents
8-hydroxyquinoline (C9H7ON) and 8-hydroxy-7-iodoquinoline-5-sulfonic acid (C9H6O4NIS) of the analytically pure grade and cerium(IV) sulfate tetrahydrate
of analytical reagent grade were used as starting materials. In all solutions, an ionic strength of I = 2 mol/L
was generated with analytical reagent grade ammonium sulfate. The initial solutions were prepared from precisely weighed portions. The content of cerium(IV) in a freshly prepared solution of cerium(IV) sulfate was determined by back titration with Mohr’s salt in the presence of ferroin5 before and after an experiment.
Instrumantal analysis
The optical density was recorded in time using a SPECORD UV VIS recording spectrophotometer equipped with a temperature-controlled cell holder for rectangular quartz cells with an optical path length l =1 cm and a KF-5 photoelectric colorimeter with a MEA-4 recording device and a temperature controlled case for standard cells with l (10.00±0.01)×10–1cm. The pH value was measured in the reaction mixture, after the recording of the optical density ((τ≈ 1 min and also
), and in the cerium(IV) solutions with a DATA METER precision pH meter. In the measurements, the concentration scale was used. The glass electrode was calibrated against HCl solutions of know concentration at I = 2, since the pHs measured did not exceed 3.0–3.5. Buffer solutions were used for the initial correction of the pH-meter scale. The instant at which the mixing vessel whereto the starting components were placed was turned upside down, was taken as the time of reaction onset, τ = 0. Kinetic measurements were carried out at 515nm, where the greatest increase in the differences
and
were observed with increasing pH (
is the initial value of the optical density of the reaction mixture at the initial time τ = 0 found by linear extrapolation of the kinetic curves in the coordinates log D–τ, where their linearity took place;
the optical density of the metal ion solution,
is the rate of its change; and
,
, is the initial rate of the redox decomposition of the complexes). The initial equilibrium concentration of the cerium(IV) complexes were determined at the instant of time τ = 0 according to formula
where
and
are the concentrations of test solutions of cerium (IV) and ligands
and
. Here and below, the line above the symbol stands for the value determined by kinetic means. The maximum value
of the rate
were found by means of the
method.6,7 The initial rate of the redox decomposition of the complexes was estimated graphically on a semi-log grid using the slope ratio of line
(Figure 1) and calculated by the linear least squares method.

Figure 1 Pseudo first-order rate curves
for the
Fer system
mol/L, I= 2,
,
nm.
Results and discussion
Composition of complexes
The metal: Ligand molar ratio in the complex formed at the time of mixing the solutions was established by the
isomolar series method
is the mole fraction of the ligand), adapted to the study of variable-valence metal complexes, and a kinetic analog of this method
where
6 Figure 2 shows that in the systems
complexes characterized by a metal:ligand ratio of 1:1 (mol/mol) are formed at the initial time. The obtained data agree with the data of kinetic studies,8 which indirectly indicate the formation of 1:1 (mol/mol) intermediate complexes in the course of oxidation of 8-hydroxyquinoline and 8-hydroxy-7-iodoquinoline-5-sulfonic acid by cerium(IV) in perchlorate medium.i

Figure 2 Diagrams: (1)
for the system
(2)
for the system
Ligand speciation
The form in which organic ligands were present in the complex was determined by analyzing the property–pH diagrams (Figure 3) by the
method. 6 The predominant form of cerium(IV) against the sulfate background
at pH studied is the monohydroxo form
7 The number of protons z displaced from the cationic form
of the molecule
and the zwitterionic molecule
by the cerium(IV) ion when equilibria
(1)
were established was estimated graphically as the slope of the dependence of
on pH,
(2)
as a result of comparison of two
data series.
Effective (depending on the pH value) stability constants
were calculated using the equation
(3)
Figure 3 demonstrate that in the course of complexation a
ion displaces two protons (z = 2) from
and
. The latter can be accompanied by the formation of chelate complexes and the closure of the 5-membered cycles.

Figure 3 Diagrams of the dependence of the logarithm of the effective stability constant of complexes on the pH. System: (1)
, (2)
Stability constants
For the complexation equilibrium with anionic species
(4)
(5)
the concentration stability constants
were calculated for each point of function
and by averaging the values obtained according to series
. Calculations were performed using the following logarithms of the cumulative protonation constants of the anionic species
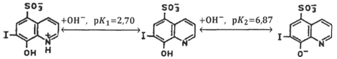
;9
10 Note that according to,10,11 the values of the dissociation constants
obtained in10 for 7-iodo-8-hydroxyquinoline-5-sulfonic acid characterize the equilibria
The confidence interval for average values of these thermodynamic characteristics, along with the optical parameters of the complexes, was calculated at a sample size of N = 14–16 with a confidence level of 0.95 using the STATOBRABOTKA statistical processing program.12 The averaged values of
determined using the method
were 15.54±0.13 for the
complex (Table 1) and 12.38±0.11 for the complex
(Table 2).
|
|
pH |
|
|
|
-0.16 |
0.13 |
0.6 |
0.12 |
2.27 |
15.83 |
-0.23 |
0.19 |
0.82 |
0.18 |
2.46 |
15.58 |
-0.28 |
0.23 |
0.9 |
0.22 |
2.57 |
15.53 |
-0.39 |
0.31 |
1.05 |
0.31 |
2.75 |
15.41 |
-0.51 |
0.42 |
1.12 |
0.4 |
2.91 |
15.43 |
-0.77 |
0.6 |
1.23 |
0.6 |
3.19 |
15.49 |
-1.28 |
1.03 |
1.43 |
1 |
3.68 |
15.58 |
-1.64 |
1.38 |
1.7 |
1.28 |
4.13 |
15.49 |
Table 1 Stability constants of the complex
determined by the
method
,
mol/L,
,
, I = 2,
,
nm, l = 1cm)*
|
|
pH |
|
|
|
-0.38 |
3.46 |
0.5 |
2.86 |
3.9 |
12.47 |
-0.38 |
4.73 |
0.6 |
2.88 |
3.91 |
12.28 |
-0.34 |
4.07 |
0.63 |
2.59 |
4.15 |
12.46 |
-0.5 |
6.2 |
0.9 |
3.79 |
4.46 |
12.23 |
-0.55 |
6.48 |
1.05 |
4.16 |
5.05 |
12.53 |
-0.55 |
6.43 |
1.12 |
4.16 |
5.05 |
12.39 |
-0.56 |
6.63 |
1.23 |
4.25 |
5.21 |
12.33 |
Table 2 Stability constants of the complex
determined by the
method
mol/L,
,
, I= 2,
,
nm, l = 1cm)*
The constancy of the calculated
value within the series
indicates that the basic equilibria in solution are taken into account correctly. Lower stability of the complex
compared to
is due to the presence of the electron withdrawing groups (sulfonic acid group and iodine atom) in the molecule
.
Conclusion
Thus, the compositions of the cerium(IV) complexes with 8-hydroxyquinoline and 8-hydroxy-7-iodoquinoline-5-sulfonic acid and also their stability constants,
=15.54±0.13 for
and
=12.38±0.11 for
, were determined spectrophotometrically and pH-metrically by the kinetic analogues of the thermodynamic methods for investigating the formation of variable-valence metal complexes at an ionic strength I = 2mol/L in a sulfuric acid medium and a temperatures of 275.15–289.15K.
Funding details
Acknowledgements
Conflict of interest
Author declares that there is no conflict of interest.
References
- Oliveri V, Vecchio G. 8–Hydroxyquinolines in medicinal chemistry: A structural perspective.Eur J Med Chem. 2016;120:252–274.
- Suwanjang W, Prachayasittikul S, Prachayasittikul V.Effect of 8–hydroxyquinoline and derivatives on human neuroblastoma SH–SY5Y cells under high glucose. PeerJ. 2016;4:e2389.
- Soroka K, Vithanage RS, Phillips DA, et al. Fluorescence Properties of Metal Complexes of 8–Hydroxyquinoline–5–sulfonic Acid and Chromatographic Applications. Anal Chem. 1967;59:629–636.
- Petrova OB, Anurova MO, Akkuzina AA. Luminescent hybrid materials based on (8–hydroxyquinoline)–substituted metal–organic complexes and lead–borate glasses. Opt Mater. 2017;69:141–147.
- Kolthoff IM, Belcher R, Stanger VA, et al. Volumetric Analysis, Vol. III ‒ Titration Methods: Oxidation–Reduction Reactions. New York: Interscience; 1957.
- Voskresenskaya O, Kinetic and Thermodynamic Stability of Cerium(IV) Complexes with a Series of Aliphatic Organic Compounds. New York: Nova Science Pudlishers, Inc.; 2013.
- Voskresenskaya OO, Skorik NA, Yuzhakova YuV. Thermodynamics of the Formation of Cerium(IV) Malonate Complex and the Kinetics of Its Redox Decomposition. Russ J Phys Chem A. 2017;91(4):627–633.
- Pondit AK, Das A, Banerjea D. Kinetics and mechanism of oxidation of 8–hydroxyquinoline and its derivatives by cerium(IV) through precursor complex formation. Trans Met Chem. 1991;16:324–327.
- Näsänen R, Lumme P, Mukula AL. Potentiometric and Spectrophotometric Studies on 8–Quinolinol and Its Derivatives. I. Ionization of 8–Quinolinol in Aqueous Solutions of Potassium Chloride. Acta Chem Scand. 1951;5:1199–1208.
- Näsänen R, Ekman A. Potentiometric and Spectrophotometric Studies on 8–Quinolinol and Its Derivatives. V. Ionization of 8–Quinolinol–5–Sulfonic Acid and 7–Iodo–8–Quinolinol–5–Sulfonic Acid in Aqueous Solution. Acta Chem Scand. 1952;6:1384–1390.
- Langmyhr FG, Storm ÅR. Complex Formation of Aluminium with 7–Iodo–8–hydroxy–quinoline–5–sulphonic Acid (Ferron). Acta Chem Scand. 1961;7:1461–1466.
- Skorik NA, Chernov EB. Computer Computations in Complex Compounds Chemistry. Tomsk: TGU; 2009.

©2018 Voskresenskay, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.





