Journal of
eISSN: 2473-0831


Mini Review Volume 5 Issue 1
Correspondence: K Szymczyk, Department of Interfacial Phenomena, Faculty of Chemistry, Maria Curie-Sk?odowska University, Maria Curie-Sk?odowska Sq. 3, 20-031 Lublin, Poland
Received: May 26, 2017 | Published: June 7, 2017
Citation: Szymczyk K, Taraba A (2017) Spectroscopic Studies of Triton X-114 and Quercetin/Rutin Solutions. J Anal Pharm Res 5(1): 00128. DOI: 10.15406/japlr.2017.05.00128
The behaviour of aqueous solutions of the nonionic surfactant, Triton X-114 (TX114) with quercetin and rutin was investigated using the UV-vis and fluorescence spectroscopic techniques. The interactions between flavonoid and the surfactant were examined in a wide range of surfactant concentration and at temperature 293K. From the obtained spectra the micropolarity of the pyrene environment in these solutions as well as the flavonoid-surfactant binding constant were determined.
Keywords: Aqueous solutions; Nonionic surfactant; Triton X-114; Fluorescence; Spectroscopic techniques; Surfactant; Flavonoid; Micropolarity; Solubilization; Liposomes; Nanocapsules
Flavonoids belong to a large group of natural polyphenol compounds and have a broad spectrum of pharmacological activities. Many natural flavonoids are characterized by extremely low solubility in aqueous media and body fluids. To improve the solubility of drugs, various methods were developed and are used in pharmaceutics: solubilization, preparation of solid disperse systems with soluble and insoluble matrices, inclusion in liposomes, nanocapsules, and others.1 Previously it was found that the solubility of the flavonoids quercetin and rutin increases substantially in the aqueous solutions of β-cyclodextrin, biopolymers, polyvinyl pyrrolidone and human serum albumin due to the formation of supramolecular complexes and that the spectral and protolytic properties of flavonoids in these organized media also change. Another type of organized media are self organized supramolecular micellar systems based on surfactants in which the solubility of hydrophobic substances can increase substantially due to solubilization.2 Thus the purpose of the presented studies was to determine the interactions between the nonionic surfactant, Triton X-114 (TX114) micelles (C=8x10-4 -8x10-3M) and quercetin as well as between the surfactant and quercetin glycoside, rutin (quercetin and rutin concentration C1 =2x10-5, 4x10-5 and 6x10-5M) by spectroscopic methods at 293K.
The intensity ratio I1 /I3 of the first (372nm) and the third (384nm) vibronic peaks of the pyrene fluorescence spectrum is related to the micropolarity of the microenvironment in which pyrene is solubilized. Therefore its variation shows a change in the hydrophobic environment with respect to composition. As follows from Figure 1 as the total concentration of TX114 increases, the I1 /I3 values display an abrupt drop to the relative stabilization at C higher than 10-3M only in the case of quercetin solutions. This abrupt sigmoid decrease in I1 /I3 clearly indicates that the aggregation of TX114 was formed, and pyrene preferentially resides in a more hydrophobic microenvironment of the aggregates. In the case of rutin solutions the I1 /I3 values decrease at C higher than 10-3M. At smaller concentration of surfactant the higher the rutin concentration the lower the values of the I1 /I3 ratio. This means that the interactions of quercetin and rutin with the micelles of the studied nonionic surfactant are quite different and as a result, also the location of fluorescence probe in the mixture changes.
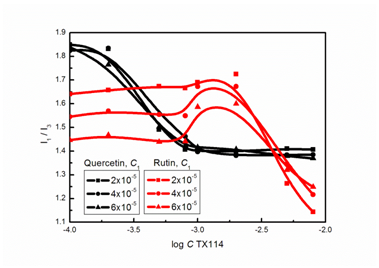
Figure 1 A plot of the I1/I3 values for aqueous solutions of quercetin and rutin at the concentration in the bulk phase equal to 2x10-5, 4x10-5 and 6x10-5 M vs. log C of TX114 at 293K
This fact is confirmed by the values of the binding constant, K, of the quercetin/rutin-surfactant system for C1 =2x10-5M calculated on the basis of the Uv-vis spectra and the relation between (A-A0 )-1 and C-1 (Figure 2).3 These values of K are equal to 1.46x103 and 0.11x103 for quercetin and rutin, respectively, and are very close to those obtained on the basis of the fluorescence emission spectra of quercetin and rutin and the relation between (I/I0 -1)-1 vs. (C-CMC)-1.4 where CMC is the critical micelle concentration of TX114.5
The interactions between quercetin and TX114 micelles are stronger than those between this surfactant and rutin molecules which was confirmed by the values of the calculated binding constant which in turn, can be applicable in the search for new ways of quercetin transportation.
None.
None.

©2017 Szymczyk, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.