Journal of
eISSN: 2473-0831


Research Article Volume 11 Issue 3
1Department of Pharmaceutical Analytical Chemistry, Al-Azhar University, Egypt
2Higher Committee of Medical Specialists, Ministry of Health and Population, Egypt
Correspondence: Ahmed Mohamed Ahmed, Assistant Lecturer of Pharmaceutical Analytical Chemistry, Poison lab, Ministry of Health and Population, Giza, Egypt
Received: August 18, 2022 | Published: October 27, 2022
Citation: Nassar MW, El-Olemy A, Emara MS, et al . Simultaneous determination of lumefantrine and artemether in the pharmaceutical preparation by capillary electrophoresis. J Anal Pharm Res. 2022;11(3):107-111. DOI: 10.15406/japlr.2022.11.00410
A sensitive, precise and accurate capillary electrophoresis method was developed for simultaneous determination of artemether and lumefantrine in pure sample and pharmaceutical formulation. Capillary electrophoresis was presented as a simple separation analytical method for the simultaneous analysis of the deliberated drugs within a shorter analytical run time. In this study, separation was achieved on fused silica capillary (30cm-50mm internal diameter), background electrolyte solution consisted of phosphate buffer (20mM, pH 6.8). The method showed to be linear (r2>0.999), precise (RSD<0.601%), accurate (recovery of 99.69% for lumefantrine and 99.96% for artemether), specific and robust. LOD and LOQ values were 1.752µg mL-1 and 5.782µg mL-1 for lumefantrine and 0.973µg mL-1 and 3.211µg mL-1 for artemether, respectively. The proposed method obtained well separation and had a perfect accuracy. The proposed method was validated according to ICH guidelines and carried out for determination of the cited drugs in their pharmaceutical formulation.
Key words: capillary electrophoresis, artemether, lumefantrine, validation, quantification
Artemether is semi-synthetic anti-malarial agent.1 It is freely soluble in acetone, soluble in tetrahydrofuran, ethanol and methanol, and virtually insoluble in water. Artemether has a molecular weight of 298.37g/mol, and molecular formula of C16H26O5 as shown in Figure 1. Lumefantrine is an aromatic fluorine derivative, which is used for the treatment of Malaria in combination with artemether.2,5 It is soluble in dichloromethane, and tetrahydrofuran, and virtually insoluble in water. Lumefantrine has a molecular weight of 528.9g/mol, and molecular formula of C30H32Cl3NO as shown in Figure 1.2 It is official in International Pharmacopoeia,3 world malaria report,4 and European Pharmacopoeia,5 Lumefantrine and artemether combination therapy is formulated as a tablets, and indicated for the treatment of acute uncomplicated malaria caused by Plasmodium falciparum, including malaria acquired in chloroquine-resistant areas.6 There are different analytical techniques applied for the determination of lumefantrine in the pharmaceutical formulation such as spectrophotometric determination,7–10 potentiometric,11 HPLC chromatographic method,11–13 GC coupled to flame ionization detector,14 and there are another available instrumental techniques for the determination of lumefantrine and artemether as a combination in the pharmaceutical formulation, the available techniques include a colorimetric method for estimation of lumefantrine and artemether,15 spectrophotometric determination,16–18 microemulsion electrokinetic chromatography,19 HPLC chromatographic method.1,20,21The aim of this study was to develop and validate a new capillary electrophoresis (CE gives better resolution than other methods. Moreover, separation processes run more efficiently and save time) method for determination of lumefantrine and artemether in pure form and its pharmaceutical formulation. The proposed method was validated in accordance with ICH guidelines Q2 (R1).22
Pure sample
Pure reference standard of lumefantrine and artemether (99.4% and 98.3 respectively, according to the reported method) were received from Egyptian international center for import.
Pharmaceutical preparation
Malari-Co® tablet (Each tablet contains 20mg of artemether and 120mg lumefantrine) was purchased from (Multi-Apex Company) the Egyptian market.
Chemicals and reagents
Apparatus
Agilent Capillary Electrophoresis 7100 System (Agilent Technologies, Germany) equipped with UV-Visible diode-array detector (190–600 nm), and Agilent chem. station software was used for data analysis.
Standard solutions
Standard stock solution (1500µgmL-1) of lumefantrine and (250µg mL-1) of artemether were prepared separately in a solvent containing tetrahydrofuran and phosphate buffer (10:90 v/v respectively). Preparation of working solutions of lumefantrine and artemether in the required concentration range was adjusted by dilution of standard stock solution with the same solvent.
Procedure
Procedures Capillary electrophoresis method Electrophoretic conditions
Construction of the calibration graph
Six different concentrations of standard solutions (20–120µg mL-1) of artemether and (30–180µg mL-1) of lumefantrine were injected into the capillary electrophoresis system. The procedure was performed in triplicate for each concentration. The analyte response obtained was plotted against the corresponding concentration of the analyte (expressed asµg mL-1).
Application to pharmaceutical formulation
Five Malari-Co® tablets were weighed and then finely powdered. Appropriate weight of powder equivalent to one tablet was accurately weighed, transferred to 100mL volumetric flask and the volume was made up to 50mL with tetrahydrofuran and phosphate buffer (10%:90% respectively). The solution was shaken vigorously for 20min then mixed for 30min and filtrated. The volume was completed with tetrahydrofuran to produce a stock solution labeled to contain 0.2mg mL-1 artemether and 1.2mg mL-1of lumefantrine. This stock solution was diluted to obtain a test sample solution containing 40µg mL-1 of artemether and 60µg mL-1of lumefantrine, and then injected into the capillary electrophoresis system.
Malari-Co is a medication used to treat malaria. It is approved for treatment of non-severe malaria. It is consisted of lumefantrine and artemether which co-formulated into one tablet. In the proposed method, Capillary electrophoresis has been described for separation and simultaneous quantitation of lumefantrine and artemether. Capillary electrophoresis provides many advantages such as fast analysis times, sensitive, minimum organic usage, and small quantities of solutions and thus overall lower consumable expenses can be attained.
Method development
Optimization of capillary electrophoresis system conditions was achieved to develop and validate a rapid and selective assay method for the determination of lumefantrine and artemether. Capillary electrophoresis technique separates ions according to their electrophoretic mobility using an applied voltage. The electrophoretic mobility depends on the radius of the atom, the viscosity, and the charge of the molecule. Capillary electrophoresis provides faster results and gives high resolution separation. It is a good technique compared to other technologies due to there is a large range of detection methods available. The capillary electrophoresis needs a careful selection of the buffer and PH to obtain the most accurate results and the best separation. Different concentrations of borate and phosphate buffers were tried at different pH values. It was found that 20mM of phosphate buffer at pH 6.8 is the optimum type at which good separation and reproducible quantification of the studied compounds was successfully done.
Phosphate buffer was tried at three different concentrations (20, 30, and 40mM) taking in account other factors constant. Phosphate buffer (20mM) provides a good resolution with adequate analytical run time. The applied voltage is directly proportional to the resolution of compounds so it can affect the efficiency of analysis. The applied voltage was progressively increased from 10 to 30 kV. The optimum resolution was obtained when applying a voltage of 15kV. Finally, the electrophoretic separation of lumefantrine and artemether was carried out under the conditions described above and the migration times were 3.18 ± 0.03 and 4.90 ± 0.04 min for lumefantrine and artemether, respectively Figure 2&3.
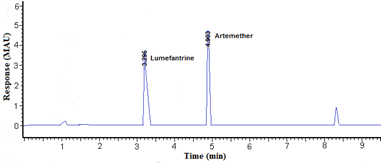
Figure 2 Electropherogram obtained for a standard solution at 60µg mL-1 lumefantrine and 40µg mL-1artemether.
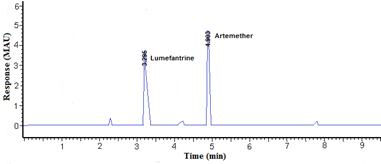
Figure 3 Electropherogram obtained for a sample solution at 60µg mL-1 lumefantrine and 40µg mL-1 artemether.
Method validation
Linearity and range
The linearity of lumefantrine and artemether was evaluated by injecting six independent levels of calibration curve in the concentration range of 20-120μgmL-1 for artemether and 30-180μgmL-1 for lumefantrine in terms of slope, intercept and correlation coefficient values. The calibration curve was prepared by plotting response verses concentration inμgmL-1 and correlation coefficient was determined as shown in Figue 4.
Intra-Day Precision (Repeatability)
The intra-day precision was calculated by analyzing three different concentrations (20, 80, 120μg mL-1) of lumefantrine and (30, 90, 180μg mL-1) artemether, three times on same day at interval of 1 hour, simultaneously and %RSD was calculated as shown in Table 1.
Inter-Day Precision (intermediate precision)
Inter-day precision was calculated daily by analyzing three different concentrations (20, 80, 120μg mL-1) of lumefantrine and (30, 90, 180μg mL-1) artemether, on three days and %RSD was calculated as shown in Table 1.
|
Parameter |
Lumefantrine |
Artemether |
|
Linearity range (µg mL-1) |
30-180 |
20-120 |
|
LOD (µg mL-1) |
1.752 |
0.973 |
|
LOQ (µg mL-1) |
5.782 |
3.211 |
|
Regression parameter* |
Y= a+ b C |
Y= a+ b C |
|
Correlation coefficient |
0.9997 |
0.9994 |
|
Slope (b) |
12797 |
19055 |
|
Intercept (a) |
-9605.5 |
-4805.5 |
|
Precision (% RSD) |
|
|
|
Repeatability |
0.601 |
0.389 |
|
Intermediate precision |
0.557 |
0.588 |
|
Robustness (%RSD) |
|
|
|
0.376 a |
0.442 a |
|
|
0.390 b |
0.392 b |
|
|
0.547 c |
0.442 c |
|
|
0.362 d |
0.576 d |
Table 1 Regression and validation data for estimation of lumefantrine and artemether by the proposed method
*Y= a + bC, where Y is the peak area and C is the concentration in µg mL-1.
a Values for three determinations with change in the pH of phosphate buffer (±0.2).
b Values for three determinations with change in the phosphate buffer concentration (±2 mM).
c Values for three determinations with change in the selected wavelength (±0.2 nm).
d Values for three determinations with change in the applied voltage (±0.2 KV).
Accuracy (% Recovery)
Accuracy of the proposed method was confirmed by recovery study from marketed preparation at three levels (80%, 100% and 120%) of standard addition. Recovery percentage of artemether and lumefantrine were calculated. Recovery percentage was found within accepted limits which achieve the accuracy of the method as shown in Table 2.
|
Drug |
Pharmaceutical taken (µg mL-1) |
Pharmaceutical Found (µg mL-1) |
Pure added (µg mL-1) |
Pure found (µg mL-1) |
%Recovery |
|
Artemether |
40 |
39.83 |
32 |
32.07 |
100.22 |
|
40 |
39.92 |
99.8 |
|||
|
48 |
47.85 |
99.69 |
|||
|
Mean ± % RSD |
99.91 ± 0.249 |
||||
|
Lumefantrine |
60 |
59.88 |
48 |
47.73 |
99.44 |
|
60 |
59.99 |
99.98 |
|||
|
72 |
72.34 |
100.47 |
|||
|
Mean ± % RSD |
99.96 ± 0.254 |
||||
Table 2 Recovery study of lumefantrine and artemether by applying standard addition technique
Limit of detection and limit of quantification
LOD and LOQ were calculated according to ICH by comparing measured signals from samples with known low concentration of analytic with those of blank samples and establishing the minimum concentration at which the analyte can be reliable detected. The analytical parameters of the proposed methods are summarized in Table 1.
Specificity
The specificity of the method was confirmed by determination of different laboratory prepared mixtures of lumefantrine and artemether in different ratios. The provided electropherograms revealed that lumefantrine and artemether were clearly completely separated from each other confirming the specificity and selectivity of the proposed method. Also the specificity was evaluated by observing possible interference from table excipients. This was achieved by the analysis of tablets where the recorded electropherograms did not show any additional peaks when compared to those of the synthetic mixture.
System suitability
System suitability test was applied to verify that an analytical method was suitable for its intended purpose; the items measured were resolution, tailing factor, and theoretical plate and all results were observed within the acceptance range, as shown in Table 3.
|
Criteria |
Results |
|
|
Lumefantrine |
Artemether |
|
|
The %RSD for five replication injections of standard preparation for artemether and lumefantrine |
0.224 |
0.262 |
|
Resolution |
1.12 |
|
|
The Tailing factor |
1.27 |
1.15 |
|
Theoretical Plates |
2974 |
2952 |
Table 3 System suitability test for artemether and lumefantrine
RobustnessRobustness of the proposed method was evaluated by subjecting the method to small variations in method condition such as phosphate buffer concentration, PH of buffer, selected wavelength, and applied voltage. Table 1 contains results of robustness in %RSD term.
Application to the finished product
The proposed method was applied successfully for determination of artemether and lumefantrine in Malari-Co® tablets. The obtained results showed absence of any interference from either excipients or additives. The results of the reported method,21 were compared with that of the proposed method. The proposed method showed good accuracy and precision for assay of lumefantrine in Malari-Co® tablets and the values were listed in Table 4.
Parameter |
Proposed method |
Reported method |
||
Lumefantrine |
Artemether |
Lumefantrine |
Artemether |
|
na |
5 |
5 |
5 |
5 |
%R |
100.1 |
100.18 |
100.26 |
100.42 |
%RSD |
0.224 |
0.262 |
0.421 |
0.441 |
SD |
0.224 |
0.262 |
0.42 |
0.439 |
Variance |
0.05 |
0.069 |
0.177 |
0.194 |
Student's t-test (2.306)b |
0.952 |
1.068 |
—— |
------ |
F-value (6.388)b |
0.284 |
0.354 |
—— |
----- |
Table 4 Results obtained after determination of Lumefantrine in Malari-Co tablet and comparison with the reported method
a Experiments number
b Tabulated values of “t “and “F” at (P = 0.05)
* Reported method: HPLC method using C18 column, mobile phase consisting of buffer and acetonitrile (40:60, v/v) and UV detector dual i.e, 210 and 303nm.21
This method is sensitive, and selective, and can be successfully applied for determination of lumefantrine and artemether the pharmaceutical preparation.
The readers can access the data represented in this research article by contacting with the author on this Email: ahmedabdrabou31@yahoo.com as the data presented is his own personal experiment and work.
The author acknowledges to staff members Pharmaceutical Analytical Chemistry Department, Faculty of Pharmacy Al-Azhar University, Cairo, Egypt for their co-operation throughout the whole work. Also acknowledgment goes to Research Laboratories, Ministry of health, Giza, Egypt for providing the necessary facilities and support in completing this work.
The authors declare that there is no conflict of interest regarding the publication of this paper.
The authors declare that there is no Funding Statement regarding the publication of this paper.

©2022 Nassar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.