Journal of
eISSN: 2473-0831


Research Article Volume 4 Issue 6
Correspondence: Mukthinuthalapati Mathrusri Annapurna, GITAM Institute of Pharmacy, GITAM University, Rushikonda, Visakhapatnam-530045, India
Received: April 26, 2017 | Published: May 11, 2017
Citation: Annapurna MM, Sushmitha M, Sevyatha VSV (2017) Simultaneous Determination of Brimonidine Tartrate and Timolol Maleate by First Derivative and Ratio Derivative Spectroscopy. J Anal Pharm Res 4(6): 00120. DOI: 10.15406/japlr.2017.04.00120
New derivative, ratio derivative and multicomponent spectrophotometric methods have been established for the simultaneous determination of Brimonidine tartrate and Timolol maleate in pharmaceutical formulations using borate buffer pH 9.0. Linearity was observed 1-60µg/ml for Timolol maleate and 1-40µg/ml for Brimonidine tartrate. The three methods were validated and can be used for the determination of Brimonidine tartrate and Timolol maleate in eye drops.
Keywords: brimonidine tartrate, timolol maleate, spectrophotometry, first derivative method, ratio derivative spectra, multicomponent mode, validation
Brimonidine (CAS No. 59803-98-4) is chemically known as 5-bromo-N-(4,5-dihydro-1H-imidazol-2-yl)quinoxalin-6-amine with molecular formula, C11H10BrN5 and molecular weight 292.14g/mol. Brimonidine (BRM) is freely soluble in water and soluble in methanol with pKa 7.78. Brimonidine is used to treat open-angle glaucoma or ocular hypertension. Brimonidine1 is indicated for the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension. Brimonidine is an α2 adrenergic agonist that acts by the activation of G protein-coupled receptor. This G protein-coupled receptor inhibits the activity of adenylate cyclase. The α2 agonist results in vasoconstriction of blood vessels and vasoconstriction reduces the aqueous humour flow.2
Timolol (CAS No. 26839-75-8) is chemically (S)-1-(tert-butylamino)-3-[(4-morpholin-4-yl-1,2,5-thiadiazol-3-yl)oxy]propan-2-ol. Timolol (TML) can be used as an antihypertensive, anti-arrhythmic, anti-angina, and anti-glaucoma agent. It has molecular formula, C13H24N4O3S and molecular weight 316.42 g/mol (pKa 9.21). Timolol3 is a beta-adrenergic antagonist and the levo isomer is the more active. Timolol is used for the treatment of migraine disorders, tremor. The combination of Brimonidine and Timolol have a rapid onset of action, with peak ocular hypotensive effect seen at two hours post-dosing for Brimonidine and one to two hours for Timolol. Very few spectrophotometric4-6 and liquid chromatographic methods7-8 are available in the literature for the simultaneous determination of BRM and TML. Generally, while doing the dissolution testing of drugs and their formulations different reagents9 of various pH are used and sometimes even first, second and fourth derivative spectroscopic methods are adopted in order to eliminate the interference from UV absorbing excipients during the analysis of formulations.10 The authors have proposed three spectrophotometric methods -first derivative, ratio derivative and multicomponent mode methods for the determination of BRM and TML in pharmaceutical formulations and the methods were validated.11
Chemicals and reagents
The stock solutions of both Brimonidine tartrate and Timolol maleate were prepared in methanol and a series of solutions were prepared on dilution with borate buffer (pH 9.0) for the construction of calibration curve. The combination of Brimonidine tartrate and Timolol maleate is available with brand name Combigan (Allergen Plc, India) as eye drops containing Brimonidine tartrate 0.2% and Timolol maleate 0.5%.
Instrumentation
UV-1800 double beam UV-VIS spectrophotometer (Shimadzu) with a pair of 10mm path length matched quartz cells is used for the study. All the sample solutions were scanned 200-400nm with medium scanning speed.
Procedure
Three spectrophotometric methods-simultaneous first derivative method (D1) (Method I), ratio derivative method (Method II) and multi-component mode (Method III) were developed for the simultaneous determination of Timolol maleate and Brimonidine tartrate.
Method I: simultaneous first derivative method (D1): The individual zero order absorption spectra of Timolol maleate and Brimonidine tartrate were converted in to their first order derivative spectra with the help of inbuilt software. The first order derivative spectra of TML shows zero crossing points at 251.5, 295.37 and 366.38 nm and that of BRM at 205.5, 234.9, 257, 308.4 and 357.5 nm. TML can be quantified from the maxima observed at 257 nm (zero crossing point of BRM) whereas BRM can be quantified from the maxima observed at 251.5 nm (zero crossing point of TML).
Method II: ratio derivative method: In ratio derivative method aliquots of TML equivalent to 1-60µg/ml were accurately transferred to 10ml volumetric flask from stock solution (1000µg/ml) and the volume is adjusted with borate buffer pH 9.0. The absorption spectra of the prepared solution were scanned and recorded in the range of 200-400nm. These solutions were added with 10µg/ml of BRM with the help of inbuilt software. The obtained spectrum is converted into first derivative with the help of the inbuilt software. The maxima of the spectrum was found to be 280nm and minima was found to be 312nm, then the amplitude was calculated. The calibration curve was constructed with amplitude on y-axis against the concentrations. Similarly, TML solutions were subtracted with 10µg/ml of BRM. The maxima of the spectrum was found to be 265.5nm and minima was found to be 312.5nm, then the amplitude was calculated. In the same way, the solutions were multiplied with 10 µg/ml of BRM. The maxima of the spectrum was found to be 278nm and minima was found to be 293nm, then the amplitude was calculated. The solutions were divided with 40µg/ml of BRM. The maxima of the spectrum was found to be 292nm and minima was found to be 313nm, then the amplitude was calculated.
In the same way, different concentrations of BRM in the range of 1-40µg/ml were scanned and recorded in the range of 200-400nm. These solutions were added with 10µg/ml of TML with the help of inbuilt software. The obtained spectrum is converted into first derivative with the help of the inbuilt software. The maxima of the spectrum was found to be 249nm and minima was found to be 264.5nm, then the amplitude was calculated. The calibration curve was constructed with amplitude on y-axis against the concentrations. Similarly, BRM solutions were subtracted with 10µg/ml of TML. The maxima of the spectrum was found to be 249nm and minima was found to be 265nm, then the amplitude was calculated. In the same way, the solutions were multiplied with 10µg/ml of TML. The maxima of the spectrum was found to be 277nm and minima was found to be 293nm, then the amplitude was calculated. The solutions were divided with 50µg/ml of BRM. The maxima of the spectrum was found to be 247nm and minima was found to be 262nm, then the amplitude was calculated. The obtained spectrum is converted into first derivative with the help of the inbuilt software. Calibration curves for both TML and BRM were constructed by plotting the amplitude values on y-axis against their corresponding concentrations.
Method III: multi-component mode: In this multi-component mode method TML and BRM were mixed in different ratios (>3) and the instrument scan (200-400nm) the solutions with the inbuilt software and finally the concentration of the individual components i.e. TML and BRM were given directly for the unknown combination of solutions (or formulation solutions). For the present study seven standard solutions containing Timolol maleate and Brimonidine tartrate were prepared in 1:10, 5:15, 10:20, 20:10, 15:30, 30:40 and 40:60 ratio Borate buffer (pH 9.0) and scanned in multi-component mode.
Method validation
Linearity: 1-60µg/ml Timolol maleate and 1-40µg/ml Brimonidine tartrate solutions were prepared from the stock solutions separately and scanned against the reagent blank i.e. borate buffer pH 9.0 and the first derivative is done with the help of inbuilt software and the absorbance values were calculated at the selected wavelengths for both the methods. A graph was drawn by taking the concentration of the drug solution on the x-axis and the corresponding absorbance values on the y-axis at the selected wavelengths.
Precision and accuracy: The intra-day and inter-day precision studies were performed at three different concentration levels (10,20 and 40µg/mL) and the %RSD was calculated. Accuracy studies were carried out for both the methods 1 and 2 (80%, 100%, and 120%) and the % recovery was calculated.
Assay of brimonidine tartrate and timolol maleate in marketed formulations: The combination of Timolol maleate and Brimonidine tartrate is available with brand name Combigan (Allergen Plc, India) as eye drops containing Brimonidine tartrate 0.2% and Timolol maleate 0.5%. The eye drops preparation was procured from local pharmacy store and extracted with methanol and then assayed after dilution with borate buffer with the three methods.
Three new spectrophotometric methods, simultaneous first derivative method (D1) (Method I), ratio derivative method (Method II) and multi-component mode (Method III) were proposed and validated for the simultaneous determination of Brimonidine and Timolol in borate buffer pH 9.0.
Method I: simultaneous first derivative method (D1)
The overlay first order derivative spectrum of Timolol maleate and Brimonidine tartrate was shown in Figure 1. Linearity was observed over the concentration range 1-60µg/mL and 1-40µg/mL for TML and BRM respectively. The linear regression equations were found to be y = 0.0014x+0.0008 (R2=0.9991) and y = 0.0054x+0.0002 (R2=0.9997) in method A and B respectively. A graph was drawn by taking the drug concentration (TML or BRM) on the x-axis and the corresponding derivative absorbance on the y-axis and a straight line graph was obtained (Figure 2A and 2B).
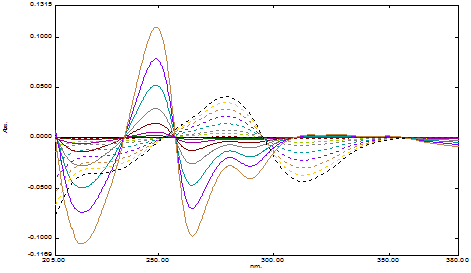
Figure 1 Overlay first derivative spectrum of Brimonidine tartrate (-----) and Timolol maleate (- - -) in borate buffer.
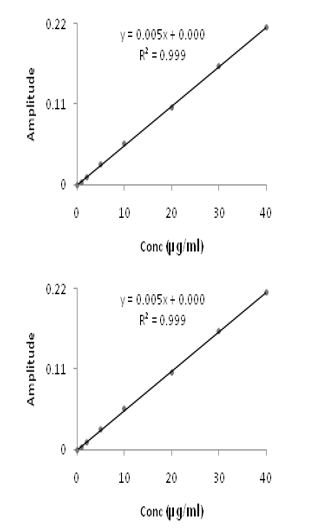
Figure 2 First order derivative calibration curves of Timolol maleate (A) and Brimonidine tartrate (B).
Method II: ratio derivative method
The overlay ratio derivative spectra of Timolol maleate and Brimonidine tartrate was shown in Figure 3 & 4. In Figure 3A-3C TML is added, subtracted and multiplied with 10µg/ml of BRM whereas in Figure 3D TML is divised with 40µg/ml of BRM. Linearity was observed over the concentration range 1-60µg/mL and 1-40µg/mL for TML and BRM for each method respectively. The linear regression equations of Timolol maleate were found to be y=0.0013x - 0.0013 (R2=0.9991), y = 0.0016x-0.0002 (R2=0.9991), y=0.0003x+0.0002 (R2=0.9994) and y=0.0037x+0.001 (R2=0.9994) in method A, B, C and D respectively. The linear regression equations of Brimonidine tartrate were found to be y=0.005x-0.0015 (R2=0.9996), y=0.0052x+0.0038 (R2=0.9992), y=0.0003x–0.0005 (R2=0.9998) and y=0.0327x-0.0004 (R2=0.9993) in method A, B, C and D respectively. A graph was drawn by taking the drug concentration (TML or BRM) on the x-axis and the corresponding derivative absorbance on the y-axis and a straight line graph was obtained (Figure 5A-5D & 6A-6D).
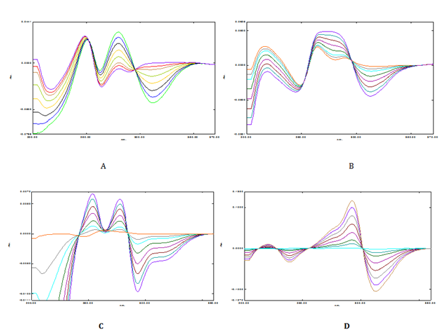
Figure 3 Ratio derivative spectrum of TML using BRM for (A) addition (B) Subtraction (C) Multiplication (D) Division.
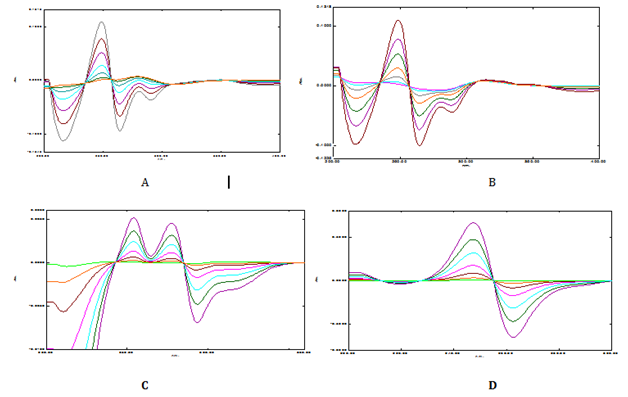
Figure 4 Ratio derivative spectrum of BRM using TML for (A) addition (B) Subtraction (C) Multiplication (D) Division.
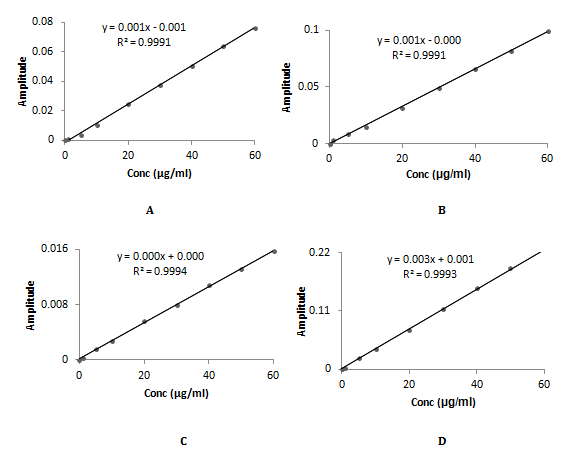
Figure 5 Calibration curve of TML using BRM for (A) addition (B) Subtraction (C) Multiplication (D) Division.
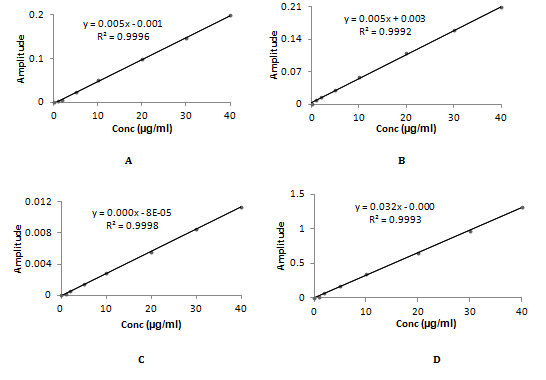
Figure 6 Calibration curve of BRM using TML for (A) addition (B) Subtraction (C) Multiplication (D) Division.
Method III: multi-component mode
In multi-component mode method TML and BRM were directly determined from the inbuilt loaded software. The eyedrop formulation (20:50) has shown BRM: TML as 20.319: 49.236 indicating that BRM is 101.59% and TML as 98.47%.
Precision and accuracy
The precision and accuracy studies were performed and the results were given in Table 1 & 2. The percentage RSD was found to be less than 2% indicating that the methods are precise and accurate. The assay of marketed formulation was found to be 98.50-99.40 Table 3).
|
Drug |
Conc (μg/ml) |
Intra-Day Precision |
Inter-Day Precision |
||||||||
|
*Conc. Obtained (µg/ml) ± SD (RSD) |
*Conc. Obtained (µg/ml) ± SD (RSD) |
||||||||||
|
[*% Recovery] |
[*% Recovery] |
||||||||||
|
Method I |
Method II |
Method I |
Method II |
||||||||
|
A |
B |
C |
D |
A |
B |
C |
D |
||||
|
TML |
10 |
9.98±0.02 (0.24) [99.8] |
9.93± 0.02 (0.18) [99.3] |
9.94± 0.02 (0.25) [99.4] |
9.91± 0.02 (0.29) [99.1] |
9.95± 0.04 (0.39) [99.5] |
9.91± 0.10 (0.99) [99.1] |
9.94± 0.06 (0.65) [99.4] |
9.92± 0.05 (0.51) [99.2] |
9.94± 0.09 (0.99) [99.4] |
9.96± 0.13 (1.37) [99.6] |
|
20 |
19.9± 0.14 (0.72) [99.6] |
19.86± 0.3 (0.54) [99.3] |
19.91± 0.05 (0.29) [99.5] |
19.89± 0.08 (0.44) [99.4] |
19.83± 0.10 (0.59) [99.1] |
19.92± 0.20 (1.05) [99.6] |
19.82± 0.19 (0.98) [99.2] |
19.92± 0.3 (0.84) [99.6] |
19.86± 0.24 (1.24) [99.3] |
19.85± 0.22 (1.14) [99.2] |
|
|
40 |
39.1±0.31 (0.09) [97.7] |
38.9± 0.31 (0.81) [97.8] |
39.2± 0.31 (0.14) [98.0] |
39.4± .31 (0.12) [98.5] |
39.1± 0.31 (0.09) [97.7] |
39.5± 0.37 (0.94) [98.4] |
39.5± 0.47 (1.20) [98.7] |
39.4± 0.35 (0.91) [98.5] |
39.5± 0.37 (0.96) [98.7] |
39.7± 0.49 (1.24) [99.2] |
|
|
BRM |
10 |
9.95±0.04 (0.39) [99.5] |
9.92± 0.02 (0.21) [99.2] |
9.94± 0.02 (0.19) [99.4] |
9.98± 0.02 (0.24) [99.8] |
9.91± 0.05 (0.51) [99.1] |
9.96± 0.13 (1.37) [99.6] |
9.93± 0.09 (0.98) [99.3] |
9.93± 0.08 (0.81) [99.3] |
9.91± 0.10 (0.99) [99.1] |
9.94± 0.12 (1.21) [99.4] |
|
20 |
19.8±0.11 (0.59) [99.1] |
19.8± 0.10 (0.52) [99.2] |
19.8± 0.08 (0.40) [99.3] |
19.9± 0.04 (0.22) [99.5] |
19.8± 0.02 (0.12) [99.4] |
19.9± 0.20 (1.05) [99.4] |
19.7± 0.21 (1.10) [98.9] |
19.9± 0.14 (0.72) [99.6] |
19.9± 0.18(0.94) [99.8] |
19.9± 0.20 (1.05) [99.6] |
|
|
40 |
39.2±0.31 (0.14) [98.0] |
39.4± 0.26 (0.67) [98.5] |
39.1± 0.31 (0.09) [97.7] |
39.5± 0.37 (0.96) [98.7] |
39.2± 0.31 (0.14) [98.0] |
39.4± 0.35 (0.91) [98.5] |
39.2± 0.31 (0.81) [98.0] |
39.7± 0.49 (1.24) [99.2] |
39.5± 0.47 (1.20) [98.7] |
39.4± 0.35 (0.91) [98.5] |
|
Table 1 Precision studies of timolol maleate and brimonidine tartrate
*Mean of three replicates.
|
Drugs |
Spiked |
Total |
Method I |
Method II |
|||
|
A |
B |
C |
D |
||||
|
%*Recovery (%RSD) |
%*Recovery (%RSD) |
%*Recovery (%RSD) |
%*Recovery (%RSD) |
%*Recovery (%RSD) |
|||
|
TML |
8 (80%) |
18 |
99.66(0.92) |
99.7(0.92) |
99.1(0.84) |
98.72(0.91) |
99.7(0.37) |
|
10(100%) |
20 |
99.19(1.07) |
99.1(1.01) |
99.5(0.93) |
99.01(0.71) |
99.4(0.64) |
|
|
12(120%) |
22 |
98.54(0.91) |
99.4(0.84) |
99.2(1.21) |
99.21(1.18) |
99.7(0.93) |
|
|
BRM |
8 (80%) |
18 |
98.88(1.16) |
98.8(1.04) |
98.8 (0.42) |
99.20(0.37) |
99.2(0.18) |
|
10(100%) |
20 |
98.61(0.98) |
99.5(1.25) |
99.4(0.67) |
99.59(0.19) |
98.4(0.48) |
|
|
12(120%) |
22 |
99.42(0.88) |
98.9(0.97) |
99.8 (0.28) |
98.10(0.67) |
98.6(0.81) |
|
Table 2 Accuracy studies of timolol maleate and brimonidine tartrate
*Mean of three replicates.
|
Formulation Brand |
Drug |
Label |
*Amount |
*% Recovery |
||
|
Method I |
Method II |
Method I |
Method II |
|||
|
Combigan Eye drops |
Timolol |
5 |
4.96 |
4.97 |
99.2 |
99.4 |
Table 3 Assay of timolol maleate and brimonidine tartrate
The three spectrophotometric methods is simple, precise and accurate for the simultaneous determination of Timolol maleate and Brimonidine tartrate in pharmaceutical formulations successfully.
The authors are grateful to M/s GITAM University, Visakhapatnam for providing the research facilities. There is no conflict of interest.
None.

©2017 Annapurna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.