Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 4
Correspondence: José Roberto Ferreira, was the advisor of Flávia Fontes Pereira Lopes in the Program of Postgraduate in Biotechnology at the Federal University of São Carlos, São Carlos, São Paulo, Brazil, Environmental Science Department, São Paulo Agribusiness Technology Agency, Postal Box 28, 13400-970, Piracicaba, SP, Brazil, Tel 5.51934E+11
Received: May 16, 2018 | Published: August 2, 2018
Citation: Lopes FFP, Ohashi TL, Mortatti J, et al. Sediment quality of the Guamium river, a small degraded catchment of the Piracicaba river basin, São Paulo, Brazil, assessed by algae toxicity assays and acid volatile sulfur contents (AVS). J Anal Pharm Res. 2018;7(4):430-435. DOI: 10.15406/japlr.2018.07.00262
The environmental quality of the Guamium river basin, a degraded ecosystem, was evaluated through sediment analysis. To this end, nine sediment samples were collected in August, 2010 (dry season) and in March, 2011 (rainy season) from the main channel and two tributaries. The AVS/SEM ratios were considered for evaluating the potential bioavailability of metals. In addition, sediment leachates were used to evaluate the toxicity of the extracts to P. subcapitata algae. During the dry season the AVS concentration (22.57µmol/g) reach values as high as those found for polluted rivers. SEM varied also with season and spatially, within 0.06 to 1.42µmol/g and 0.28 to 1.94µmol/g in the dry and rainy seasons respectively, leading to different potential availability of metals to the sediment along the basin. When growth differences of algae in sediment leachates were compared to the control (distilled water), by using Tukey test (p≤0.05), significant differences were obtained for P3 (0.02*), P5 (0.02*) and P7 (0.04*), agriculture areas in the dry season, for P1 (0.01*), a source area, and for P6 (0.01*) and P7 (0.01*), in the agriculture zone for the rainy season. The mismatches of these two indices of sediment quality denote that other parameters are involved in this ranking, such e.g. pH, other metallic cations, pluviometry, river discharge, alloctone material inputs and xenobiotic molecules. Anyway, the toxicity test to the algae P. subcapitata is the resultant of all abiotic effects over the biota interacting together, thus reflecting alterations from the natural conditions of the studied ecosystem.
Keywords: Guamium river basin, sediment toxicity, microalgae, AVS/SEM, land use, sugarcane and energy
Energy consumption in the world has grown exponentially during last decades due to the pronounced population growth and industrial development. This demand has been mainly supplied by fossil fuels, yet burning contributes to the increase in atmospheric CO2 concentration, an important aspect concerning the global warming.1–3 Other environmental impacts have been associated with this process. Consequently, alternative energy sources, preferably renewable and less polluting, such as ethanol, are being sought, aiming to reduce CO2 emission towards the environment.4,5 The replacement of gasoline by ethanol of sugar cane, reduces up to 73% of the CO2 emissions.5 On the land use point of view, it is observed that one hectare of sugar cane yields 4,420 kg CO2 per year.6,7 So, the production of fuels derived from biomass is not totally free in terms of environmental impact. Aquatic systems contamination is one of the most important consequences of these activities, leading to the degradation of water quality, and compromising their use for both, human consumption and aquatic life preservation. A relevant aspect of this deterioration is the eutrophication resulting from high input of nutrients derived from fertilizers8,9 and the resulting toxicity of the active ingredients with complex organic molecules, such as herbicides frequently applied to crop lands.10
In aquatic systems, most of contaminants accumulate in the sediments, from where they can be released to the water column according to changes in the physicochemical conditions at the sediment-water interface. Thus, the sediment acts as a sink and source of chemical species in such environment,11 being an useful compartment to assess for the evaluation of the degree of impairment of water bodies in terms of land use and watershed characteristics.
As a result of microbiological activity, diagenetic process associated with the depletion of the dissolved oxygen concentration in the sedimentary environment may occur. According to Rabouille and Gaillard12 and Di Toro et al.,13 sulfate is the major electron acceptor driving organic matter oxidation in anaerobic sediments. The generated sulfide in the process of organic matter oxidation, the volatile acid one (AVS), is an important ligand for controlling bioavailability/toxicity in the aquatic environment,14 mainly concerning to the fate of the Simultaneous Extracted Metals–SEM.15
In the present work, the sediment quality of the Guamium river basin along its entire main channel and two tributaries, a landscape supporting nearly 80% of its total area with sugarcane plantation, was evaluated on a seasonal approach, by knowing aspects of the potential toxicity of this compartment. Therefore, the AVS/SEM ratios were estimated and the chronic toxicity tests were carried out as well, by using the Pseudokirchneriella subcapitata chloroficea algae (Korshikov) F. Hindák, belonging to the 1st trophic level of the aquatic food chain.
Study area
This work was carried out at the Guamium river basin (7,051ha), Piracicaba, São Paulo, Brazil, with 78% of its total area occupied by sugarcane crop (5,477ha), contrasting with 491ha only of remaining Atlantic Forest. The main soil types in the basin are the latosoils and argisoils. Riverine drains a small urban area upstream, an agriculture one in the middle and a commercial and industrial one downstream (Figure 1).
The total monthly precipitation for a whole year, including the sampling periods (August, 2010 and March, 2011) were obtained from the APTA Metereological Station located at sampling point P7 (Figure 1). During sampling campaign, river water physicochemical variables, such as pH, DO, TDS, temperature and conductivity were in situ estimated, 20cm below the water surface, by using a multi-sensor HANNA HI 9828 probe (Texas, USA).
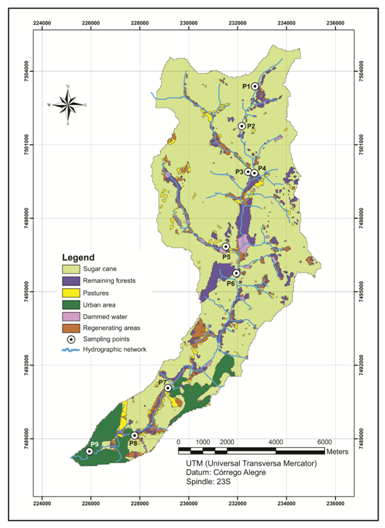
Figure 1 Sampling points and land use at the Guamium River hydrographic basin. (Supplied by BioGeoTec Searches and Environmental Solutions Ltd).
Sediment sampling
Chemicals of analytical reagent grade were used throughout. Sediment samples were collected (Figure 1) in the main river channel and two other tributaries (P3 - Agua Branca and P5-Duas Aguas) from upstream towards downstream, in August, 2010 (dry season) and March, 2011 (rainy season), with a total of nine sampling points.
Bottom sediment was obtained with a 1.0L stainless steel sampler. These samples were used for AVS and SEM determinations. For toxicity bioassays, samples were stored at 4°C inside a plastic box with lid until processing.
AVS and SEM determinations
AVS were estimated with the procedures proposed by Mortatti et al.,16 based on the concepts presented by Di Toro et al.,17 and Allen et al.18
Wet sediment samples (1.0-5.0g) were extracted in a cold 2.0mol/l HCl solution. At the end of the process, the residual sediment plus the extractant were removed from the volatilization/extraction balloon and filtered through a glass fiber filter, previously dried at 60°C until constant weight. SEM was quantified by a model 3000DV inductively coupled argon plasma optical emission spectrometer (ICP-AES, Perkin Elmer Optima, Ohio, USA). Both AVS and SEM values were expressed as µmol/g dry sediment mass. The potential bioavailability of metals in sediments was estimated by the ratio AVS/SEM. Values higher than 1.0 indicate low metals bioavailability due to sulfur complexation, whereas values lower than 1.0 indicate higher availability of metals, as metals remain free in the aqueous medium.
Sediment bioassays
Sediment toxicity tests were accomplished with Pseudokirchneriella subcapitata a clorophycean microalgae. The leachates were obtained by adding 200mL of distilled water to 50g of wet sediment samples. The extractions were performed in 250mL Erlenmeyer flasks, by a mechanical gently shake for a 24h period. For particle settlement an additional 24h resting period was performed by keeping samples in the refrigerator.19 Afterwards the leachates were carefully transferred to non-toxic plastic containers, and stored in a refrigerator to be used in the toxicity tests without any further dilution. Assays were carried out in triplicate.
The P. subcapitata inoculum was obtained at the Laboratory of Physiology and Biochemistry of Algae, Botany Department, Federal University of São Carlos. The algae was asseptically cultured in a previously autoclaved (120ºC, 1.0atm, 20min) CHU-12 medium.20 The culture was grown 25±1ºC, under gentle aeration, keeping a 12:12h light:dark period. New cultures were kept in 250mL Erlenmeyer flasks with 150mL of distilled water plus 3.0mL of a concentrated stock solution of CHU 12 medium, in a growth chamber (FANEM trade, NT-708 model, São Paulo, Brazil), under controlled conditions of temperature (25ºC±2ºC) and photoperiod as specified above.
For the chronic toxicity tests, 5.0mL of P. subcapitata in the exponential growth phase were added to the test recipients consisting of 250mL, non-toxic plastic vessels with 50mL of sediment leachate. The recipients were kept under continuous illumination through fluorescent light bulbs (around 4500lux) at 25ºC±2ºC, under constant aeration supplied by a compressed air and additional manual shaking, three times a day. These chronic tests were carried out in triplicate during a period of 120h. At the end, 1.0mL aliquots from each experimental flask were taken for cell counting in the improved Neubauer Chamber by using an optical microscope. The number of cells counted was used to calculate the algal culture densities.
To assess for the sediment leachates toxicity to the algae Pseudokirchneriella subcapitata, for samples collected in August 2010 and March 2011, the program BioEstat version 5.0 was used for the One Way Analysis of Variance (ANOVA) with the parametric Tukey test at significance level of p≤0.05. Data evaluation took in to account the growth differences of algae observed between the sediment leachates and control (distilled water).
In order to contribute to the environmental data information, other than CO2 emission to the atmosphere and river water quality in the sugarcane crop production chain, the Guamium hydrographic basin, which occupies nearly 80% of its drainage area cover with this plant was investigated. In this paper, bottom sediment was exploited for its potential bioavailability of metals and toxicity for the microalgae P. subcapitata. The basin pluviometry and physicochemical variables of the river water in a sazonal approach were also considered.
Pluviometry
The monthly pattern of precipitation from August, 2010 to July, 2011, including the sampling periods, is presented in Figure 2. No precipitation occurred in August, 2010, whereas 251.8mm of rain fell over the Guamium river basin during March, 2011. For the whole year a total of 1,656.7mm of rain was recorded.
Physicochemical data of river water
Guamium river water physicochemical data were obtained during two sampling campaigns, August, 2010 and March, 2011 (Table 1). On Table 1, EC is expressed in µS/cm. Both, DO and TDS concentrations are expressed in mg/l. In the dry period temperature varied from 18 to 20.80C, with an average of 19.480C, while in the wet season, from 23 to 25.70C and average of 24.30C. The pH in the dry period varied from 5.64 to 7.15 and from 5.13 to 6.68 in March. The DO variation was from 1.3 to 7.9mg/l in the dry period and from 5.3 to 8.8mg/l in the rainy one. The EC, varied from 16 to 545µS/cm in the dry period to 11 and 326µS/cm in the wet one. TDS varied from 8.0 to 273mg/l in the dry period to 6.0 to 163.0mg/l in the wet season.
Sampling |
2010 |
2011 |
2010 |
2011 |
2010 |
2011 |
2010 |
2011 |
2010 |
2011 |
Points |
0C |
0C |
pH |
pH |
DO |
DO |
EC |
EC |
TDS |
TDS |
1 |
20.8 |
23.5 |
5.64 |
5.23 |
5.3 |
5.0 |
16.0 |
11.0 |
8.0 |
6.0 |
2 |
18.0 |
23.0 |
5.81 |
5.13 |
2.4 |
5.5 |
40.0 |
24.0 |
20.0 |
12.0 |
3 |
18.7 |
23.5 |
5.80 |
5.19 |
1.3 |
5.3 |
60.0 |
33.0 |
30.0 |
16.0 |
4 |
18.3 |
24.0 |
6.10 |
5.28 |
3.1 |
5.8 |
49.0 |
28.0 |
25.0 |
14.0 |
5 |
20.5 |
24.3 |
6.62 |
6.46 |
3.6 |
8.0 |
545.0 |
326.0 |
273.0 |
163.0 |
6 |
20.7 |
25.7 |
6.59 |
6.68 |
5.9 |
8.6 |
168.0 |
74.0 |
84.0 |
37.0 |
7 |
19.4 |
24.6 |
7.01 |
6.12 |
7.9 |
8.5 |
157.0 |
80.0 |
79.0 |
40.0 |
8 |
19.3 |
25.0 |
6.74 |
6.6 |
7.1 |
8.1 |
161.0 |
80.0 |
80.0 |
40.0 |
9 |
19.7 |
25.4 |
7.15 |
6.47 |
6.6 |
8.8 |
193.0 |
90.0 |
97.0 |
45.0 |
Table 1 Physicochemical data of river water obtained in situ, 20cm below surface, at the sediment sampling points
AVS and SEM
Data on AVS and SEM for the sediment samples collected in the dry and rainy seasons are presented in Table 2 & Table 3 respectively. In a first glance it can be seen a lack of data in the AVS/SEM columns, being four in Table 2 (P2, P6, P7 and P8) and three in Table 3 (P1, P6, P8). In all situations, AVS were below the method detection limit-LOD. Consequently, it was not possible to calculate the AVS/SEM ratios for the sediment samples collected at these sampling points, being difficult to quantify the real variations of this index in both seasons.
Sampling |
Dissolved metals (µmol/g) |
ΣSEM |
AVS |
AVS/SEM |
|||||
Zn |
Co |
Cu |
Ni |
Cd |
Pb |
||||
P1 |
< |
< |
0.02 |
< |
< |
0.04 |
0.06 |
0.38 |
6.3 |
P2 |
< |
0.14 |
0.06 |
< |
0.26 |
0.10 |
0.56 |
< 0.01 |
0.179* |
P3 |
< |
0.06 |
0.14 |
< |
0.04 |
0.10 |
0.34 |
0.81 |
2.4 |
P4 |
< |
0.02 |
0.06 |
< |
0.02 |
0.04 |
0.14 |
0.11 |
0.78 |
P5 |
< |
0.2 |
0.08 |
< |
0.02 |
0.06 |
0.36 |
22.57 |
62.69 |
P6 |
< |
0.32 |
0.88 |
< |
0.08 |
0.14 |
1.42 |
< 0.01 |
0.007* |
P7 |
< |
0.18 |
0.36 |
< |
0.02 |
0.10 |
0.66 |
< 0.01 |
0.015* |
P8 |
< |
0.20 |
0.38 |
< |
0.04 |
0.14 |
0.76 |
< 0.01 |
0.013* |
P9 |
< |
0.12 |
0.48 |
< |
0.04 |
0.16 |
0.80 |
1.83 |
2.48 |
Table 2 AVS (µmol/g), SEM (µmol/g) and AVS/SEM ratios in bottom sediment samples of Guamium river collected in August 2010.
< = LOD.
Zn: 0,05-0,1mg L-1, Ni: 0,05mg L-1, Co: 0,05mg L-1, Cd: 0,05mg L-1
*Data ed for the ratio AVS/SEM based on the LOD (0.01)
In Table 3 one can see the results obtained for the analysis of AVS, SEM and the AVS/SEM ratios for bottom sediment samples collected in March 2011. For this season SEM varied from 0.28 to 1.94, in a wider range than that observed in the dry period, being the two limit values higher than those obtained in August, 2010.
Sampling |
Dissolved metals (µmol/g) |
ΣSEM |
AVS |
AVS/SEM |
|||||
Zn |
Co |
Cu |
Ni |
Cd |
Pb |
||||
P1 |
< |
0.04 |
0.14 |
< |
0.02 |
0.08 |
0.28 |
< 0.01 |
0.035* |
P2 |
< |
0.06 |
0.10 |
< |
0.02 |
0.16 |
0.34 |
1.19 |
3.72 |
P3 |
< |
0.06 |
0.52 |
< |
0.02 |
0.18 |
0.78 |
0.15 |
0.20 |
P4 |
< |
0.06 |
0.14 |
< |
0.02 |
0.08 |
0.30 |
0.72 |
2.77 |
P5 |
< |
0.38 |
0.30 |
< |
0.02 |
0.16 |
0.86 |
0.01 |
0.01 |
P6 |
< |
0.18 |
0.18 |
< |
0.04 |
0.10 |
0.50 |
< 0.01 |
0.02* |
P7 |
< |
0.12 |
0.20 |
< |
0.02 |
0.08 |
0.42 |
0.44 |
1.04 |
P8 |
< |
0.62 |
1.02 |
< |
0.10 |
0.20 |
1.94 |
< 0.01 |
0.005* |
P9 |
< |
0.10 |
1.26 |
< |
0.10 |
0.16 |
1.66 |
0.15 |
0.09 |
Table 3 AVS (µmol/g), SEM (µmol/g) and AVS/ΣSEM ratios in bottom sediment samples of Guamium basin collected in March 2011
< - LOD.
Ni : 0,05mg L-1, Zn: 0,05-0,1mg L-1
*Data calculated for the ratio AVS/SEM based on the LOD (0.01)
Toxicological tests
Cell density: The aim of these assays was to evaluate the algae growth when exposed to 50mL of sediment leachates obtained from the sampling points of the Guamium river basin. The lower the growth of the organism exposed to the sediment leachate in comparison to the control treatment (distilled water), the higher the toxicity of the sediment. The average cell densities of P. subcapitata exposed to sediment leachates and controls are presented in Figure 3 & Figure 4.
Pluviometry
In average for the study area the rainy season occurs between December and April. The pluviometry increases the river discharge and, depending on the basin structure and characteristics, may lead to resuspension of bottom sediments, altering the bioavailability conditions of adsorbed metals.21 Differences upon water quality have already been reported by the wet season influence in a sugarcane field area,22 like observed in this paper for the Guamium hydrographic basin. In these conditions, rainfall enhances the surface run-off, whose water infiltration depends on the soil type.23
Physicochemical data of river water
It is well known that the hydrographic basin water quality is linked to the land use.22–26 In this approach it was taken into account that water quality depends on many variables, like soil types, order of the basin, width and kind of riparian vegetation, rainfall rate, topography, etc,23,26–28 which are difficult to match among different landscapes. However it constitutes an useful tool for monitoring data information in a historical series studies,28 which consider the spatial land use variation with time in the same basin.22,27 Because of that, Table 1 was built. There, it can be seen values for pH, temperature, Dissolved Oxygen–DO, Electrical Conductivity-EC and Total Dissolved Solids-TDS for the nine sampling points along the basin, including the main channel and two tributaries, for both seasons, dry and rainy one. The Duas Aguas tributary (P5) differs from the other sampling points in the basin, presenting the highest values for TDS and EC in both seasons. In general, the lowest values for these parameters, were obtained at the headspring, increasing downstream towards the mouth of the river, an urban area, where some industrial activities are developed. Through these parameters, it was possible to observe that in the rainy season, dissolved ions were diluted as the river discharge increases. This is a characteristic of the ecosystem, and depends on the landscape; degree of preservation of the surrounded area and the water residence time in the basin among others.16 The Guamium basin has only 491ha of remaining Atlantic Forest, from its total drainage area (7,051ha). The river discharge plays a positive influence on the DO concentration. Dissolved oxygen reached very low concentrations in the dry period, which could even impair the minimum required conditions for the aquatic life, as observed from P2 to P5, which include the two tributaries. Surprisingly, previous works carried out in the Guamium basin indicated a good index of fish biological integrity – IBI. Considering a spatial approach, fish community was not affected by agriculture land use. Instead of it, at downstream, where the industrial activities occur, the IBI was decreased.29,30 The temperatures were quite different between the two seasons, as expected for winter (August, 2010) and summer (March, 2011), and in both seasons, the lowest values were not obtained at the headspring, or headwater, as should be expected for such areas in a preserved stream due to the boarder plant protection.22,31 The pH range indicates not so high variations between the periods, denoting a buffering capacity of the entire aquatic system.
Regarding environmental aspects, the sugarcane land use has not only been investigated for the surface water quality as described above and groundwater,32 but also with the aim to assess for its influence on the occurrence and distribution of fish and microinvertebrates in a water body,20,22,24,33 even for the Guamium basin ichthyofauna.29,30 However, there is little or no data on an approach to correlate the use of sugarcane land with river sediment as an indicator of pollution, exploiting its potential for metal availability and biota toxicity.
AVS and SEM
AVS/SEM ratios were considered in a spatial approach. Such protocols are well established for this compartment quality evaluation, in a wide range of proposals.34 Based on the concept of the role of sulfur complexation for metals, it is possible to realize that as low as the AVS/SEM ratios were lower than 1, the higher is the metal availability in the sediment, increasing its potential toxicity. With Table 2 & Table 3, by using the AVS–LOD (0.01µmolg-1), it is possible to estimate how far they are from 1. By example, the AVS/SEM ratios for P6, P7 and P8 were 0.007, 0.015 and 0.013 respectively. So, this index is still useful in a qualitative approach.
The AVS concentrations in Guamium basin sediments varied in a wide range, being particularly high at Agua Branca and Duas Aguas tributaries with values ranging from 0.01–22.57µmol/g. These values are comparable with ranges reported for the highly polluted Tietê river, SP, Brazil) (0.2–28.8µmol/g)18 and for the Dommel river, The Netherlands (0.01–22.45µmol/g).35 It can then be observed, based on AVS properties, that a spatial variation on Guamium river sediment toxicity can be expected.
For the dry season, like occurred for AVS, SEM varied in a quite wide range, from 0.06 to 1.42µmol/g, indicating how different were the metal concentrations in sediments in a spatial variation at the Guamium river basin, as a function of land use. Among metals, Zn and Ni concentrations for all samples were below the LODs of the method. In general, upstream (P1–P5), the concentrations of SEM were lower than those obtained downstream (P6–P9). On the other hand, AVS concentrations were high at P3 and P5, the two tributaries of the Guamium river, and at P9, resulting in values higher than 1 for AVS/SEM ratios in these points. So, metals availability can be higher downstream, in which the AVS/SEM were lower than 1, with exception at P9, an industrial area. AVS concentration was particularly high at P5, the Duas Aguas stream, presenting the highest value for the AVS/SEM ratios in both sampling period. According to Table 2, the lowest SEM value was obtained for P1, at riverhead. The SEM concentration patterns were as follow: P4 < P3 < P5 < P2 < P7 < P8 < P9 < P6.
By the data presented in Table 2 & Table 3, sediment potential toxicity varied with season along all sampling stations with a systematic tendency of alternating ratios higher than 1 in rainy period to ratios lower than 1 in the dry period and vice versa. With exception of P9 at dry period, low AVS/SEM ratios were observed in both sampling campaigns from P5 to P9, denoting higher bioavailability of metals towards river downstream. Sediment sample from the Duas Aguas stream has change drastically the AVS/SEM ratio, from 62.69 to 0.01 and neither Zn or Ni were detected in the samples again.
Toxicological tests
Cell density: Based on Figure 3, cell densities show different degrees of toxicity along the sediment samples leachates, when compared to the control. At P1, P3 and P9, they were even higher than the controls. In general the algae growth is being reduced between P1 and P8, increasing again in P9. Very low growth were obtained at P5, P6 and P8 in the middle and low river, an agriculture area. By the Tukey test (p≤0.05), significative algae growth differences with the controls were observed for the sampling points P3 (0.02*), P5 (0.02*), respectively, the Agua Branca and the Duas Aguas tributaries and P7 (0.04*). At P3, instead of toxicity, this difference was positive for algae cells growing at the sediment sample leachate from the Agua Branca stream, probably due to the input of nutrients, like P and N to the system, which enhance the abiotic conditions for primary producers36, even taking into account the AVS/SEM ratio of 6.3, which is a score for avoiding potential toxicity. Both P and N occur in river water mainly in anionic forms, such as PO43-, NO3-, NO2-, which are not complexed by sulfur. This can also be considered an indirect demonstration of the eutrophication of the aquatic system. At P5, although the AVS/SEM ratio was so elevated, being the highest one of all collected sediment samples, the toxicity to the algae community was high. This is a typical situation in which other cations than those considered in the simultaneously extracted metals analysis are presented and available in the sample. The sediment from P7 was also toxic to the organisms and the AVS/SEM ratio was very low, constituting another agreement with the concept of the potential toxicity of metals in sediments. No significant effects on algal growth occurred in the remaining points, although increases and decreases in cells growth when compared to the controls were observed (Figure 3).
For the rainy season (Figure 4), another pattern of organism responses was observed for the toxicity tests, as important differences in cell densities were observed for this period in comparison to the previous one. So we could pointed out that the sediment sample from P1 (0.01*), a source of the basin, was very toxic. This is an important data to reinforce the degree of degradation of this ecosystem. From P2 to P4 a positive growth of organisms was observed, without any toxicity at P5, which presented a stable situation in comparison to the control. Sediment samples were toxic from P6 to P9, as expected for the obtained AVS/SEM ratios in these respective sediment samples. These statements were also confirmed by the Tukey test (p≤0.05) for sampling points P6 (0.01*) and P7 (0.01*), both located at an agriculture zone in the basin.
The only sediment sample classified as toxic for the two sampling campaign, dry and wet periods, was P7. In both cases AVS/SEM ratios were very low in the dry period and slightly higher than 1 in the wet one. As discussed previously in this section, after an increase in the river discharge caused by the rain water, another conditions could arise in the sediment water interface. In addition, the input of alloctone materials and run-off corroborate for a higher toxicity conditions in the sediments.37Sediments from the upper portion of the Guamium river basin, (P2 to P4), from the tributary Duas Aguas (P5) and from the lower course (P8 and P9) did not significantly affect the growth of P. subcapitata (Figure 4). This strong change between seasons can be related to the basin response to the pluviometry due it small size and unprotected marginal area surrounded.38
Beside AVS as a major agent controlling cations availability to the biota, other xenobiotic potential inputs can occur in the Guamium river basin, like herbicides used in the sugarcane crop. Previous data indicated high As concentrations, as As3+ and As5+ in the sediments of the Guamium basin.39Arsenium is an active component of the Volcane (Sodium hydrogen methylarsonate-MSMA) a concentrate soluble herbicide molecule (CH4AsNaO3).40 Arsenium lethal concentrations to chlorophyte and cyanophyte algaes were already reported in a wide range of concentrations, being the toxicity highly function of element speciation.41 Arsenium is not considered in the SEM concentrations, which could be one of the reasons for not match the AVS/SEM data with bioassay toxicity, mainly taking in to account that the reactive As co-precipitate with AVS-acid volatile sulfides.42
Nonetheless, other aspects can be involved for the obtained results. It should also be considered the role of sediment as sink/source as a function of pH and redox conditions of the medium43,44 and sediment particles composition for controlling the adsorption/desorption of dissolved cations.45 Finally, the obtained data are of importance to be present considering the scarcity of information on environmental aspects of an agriculture area devoted to sugarcane cultivation, a renewable fuel source.
By using the sediment to assess for the ecosystem quality at the Guamium river basin, based on AVS/SEM ratios and the toxicity to the algae P. subcapitata, a primary producer in a web food chain, many aspects of the basin land use could be revealed. Although these different observations didn’t match in hundred per cent of the results, most of the presented data were linked to each other, providing a good indications on the degree of quality of this substrate. This, even not considering the whole of organic matter for complexing cations in the aquatic system and some low values obtained for the AVS in the sediment samples collected at the Guamium river. Through the concentration values obtained for the SEM it was possible to realize how each specific metal concentration was in comparison to a higher polluted environment. This approach can be applied for the AVS data as well. The seasonality has a significative influence on the AVS and SEM obtained data. This change between seasons can be related to the basin response to the pluviometry due to the small size and unprotected marginal area surrounded. This can explain how different were the metal concentrations in sediments in a spatial variation at the Guamium river basin, as a function of land use. The only observed significative toxicity for both seasons were at P7, an agriculture area. Both, the AVS/SEM ratios and toxicity bioassays test can be used as an indicative data to assess for the sediment quality in an ecosystem. Sugarcane production brings alterations in the Guamium river sediment, decreasing its quality.
Our gratitude to Fundação de Amparo à Pesquisa no Estado de São Paulo–FAPESP for providing the Scholarship to Flávia Fontes Pereira Lopes, Process n0: 2009/13331-0 and to Prof. Odete Rocha from UFSCAr for her valuable support and scientific suggestions.
The author declares that there is no conflict of interest.

©2018 Lopes, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.