Journal of
eISSN: 2473-0831


Review Article Volume 7 Issue 4
Correspondence: Rishikesh Gupta, Institute of Pharmacy, Bundelkhand University, Jhansi (UP)-284128, India, Tel -9450040476
Received: February 02, 2018 | Published: July 6, 2018
Citation: Gupta R, Tripathi P, Bhardwaj P, et al. Recent advances in gastro retentive drug delivery systems and its application on treatment of H. Pylori infections.J Anal Pharm Res. 2018;7(4):404-410. DOI: 10.15406/japlr.2018.07.00258
Oral absorption of drugs with narrow absorption window in the upper small intestine shows poor bioavailability with conventional dosage forms due short residence time. To overcome this restriction and to increase the bioavailability of these drugs, controlled drug delivery systems with a prolonged residence time in the stomach can be used. Gastric retention drug delivery system can be used to prolonged residence times of the drug in the upper part of the gastrointestinal tract. The current review deals with formulation developments of gastric retentive drug delivery systems which is prolonged residence time and enhancing oral bioavailability of the drugs. This review discusses on gastroretentive delivery system especially designed against H. pylori, including patented delivery systems and marketed products, and their advantages and future potential for gastric retention drug delivery systems are discussed.
Keywords: H. pylori, floating system, bioadhesive system, gastroretentive drug delivery system
In recent years, scientific and technological advancements have been made in the research and development of rate-controlled oral drug delivery systems by overcoming physiological adversities, such as short gastric residence times and unpredictable gastric emptying times.1,2 The oral bioavailability of drugs with an absorption window in the upper part of the gastro intestinal tract is generally limited with conventional dosage forms such as tablet, capsules and granules. These drugs can be delivered ideally by slow release from the stomach to give a localized effect at the site of action.3,4 Improved efficacy is expected for drugs that are used in the treatment of gastric disorders like ulcers and H. pylori infections.5 Many drugs categorized as once-a-day delivery have been demonstrated to have suboptimal absorption due to dependence on the transit time of the dosage form, making traditional extended release development challenging. Therefore, a system designed for longer gastric retention will extend the time within which drug absorption can occur.6
Gastric emptying of dosage forms is an extremely variable process and ability to prolong and control the emptying time is a valuable asset for dosage forms, which reside in the stomach for a longer period of time than conventional dosage forms. Several difficulties are faced in designing sustained release systems for better absorption and enhanced bioavailability.7 The residence time is main factor to limit the absorption of drug in the stomach and upper intestine. It also modified in vitro & in vivo release profile of the oral conventional dosage form. To overcome this restriction and to increase the bioavailability of these drugs, sustained drug delivery systems, with a prolonged residence time in the stomach, can be used.8
Gastro-retentive dosage forms (GRDFs) are designed to be retained in the stomach for a prolonged time and release their active ingredients and thereby enable sustained and prolonged input of the drug to the upper part of the gastrointestinal (GI) tract. This technology has generated enormous attention over the last few decades owing to its potential application to improve the oral delivery of some important drugs, for which prolonged retention in the upper GI tract can greatly improve their oral bioavailability and/or their therapeutic outcome.9
Gastro retentive delivery systems are designed to be retained in the stomach for a prolonged time and release their active ingredients and thereby enable sustained and prolonged input of the drug to the upper part of the gastrointestinal (GI) tract. This technology has generated enormous attention over the last few decades owing to its potential application to improve the oral delivery of some important drugs for which prolonged retention in the upper GI tract can greatly improve their oral bioavailability and/or their therapeutic outcome. Gastro retentive delivery system can be classified as follows.
Bioadhesive systems
Bioadhesive drug delivery systems are used as a delivery device within the lumen to enhance drug absorption in a site specific manner (Figure 1). This approach involves the use of bioadhesive polymers, which can adhere to the epithelial surface in the stomach.10 Bioadhesive systems adhere to gastric epithelial cells or mucous and extend the gastric retention by increasing the intimacy and duration of contact between gastro retentive drug delivery system (GRDDS) and the biological membrane. Some of the most promising excipients that have been used commonly in these systems include polycarbophil, carbopol, lectins, chitosan and gliadin and alginate etc.
Bioadhesive systems are those which bind to the gastric epithelial cell surface or mucin and serve as a potential means of extending the gastric residence time of drug delivery system in the stomach, by increasing the intimacy and duration of contact of drug with the biological membrane. The surface epithelial adhesive properties of mucin have been well recognized and applied to the development of GRDDS based on bioadhesive polymers. The ability to provide adhesion of a drug to the mucous layer provides a longer residence time in a particular organ site, thereby producing an improved effect in terms of local action or systemic effect.
Expandable systems
Expandable gastric retentive delivery systems are easily swallowed and reach a significantly larger size in the stomach due to swelling or unfolding processes that prolong their gastric retention time.11 After drug release, their dimensions are minimized with subsequent evacuation from the stomach. Gastro-retentivity is enhanced by the combination of substantial dimensions with high rigidity of the dosage form to withstand the peristalsis and mechanical contractility of the stomach. Narrow absorption window drugs compounded in such systems have improved in vivo absorption properties.
Expansion mechanism of this system is swelling to an extent that prevents their exit from the pylorus. As a result, the dosage form is retained in the stomach for a long period of time. These systems may be named as “plug type system”, since they exhibit the tendency to remain logged at the pyloric sphincter if that exceed a diameter of approximately 12-18mm in their expanded state. The formulation is designed for gastric retention and controlled delivery of the drug into the gastric cavity. Such polymeric matrices remain in the gastric cavity for several hours even in the fed state. A balance between the extent and duration of swelling is maintained by the degree of cross-linking between the polymeric chains. A high degree of cross-linking retards the swelling ability of the system maintaining its physical integrity for prolonged period. The following schematic presentation (Figure 2) explained the mechanism of expandable drug delivery system.
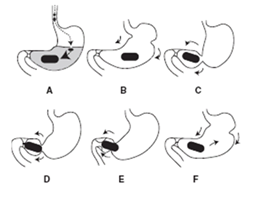
Figure 2 Represents expandable drug delivery system. A) The device significantly swells on contact with gastric fluids (to a few hundred times of the original volume); B – D) the gastric contraction pushes the hydrogel to the pylorus; E) the gastric contraction slips over the surface of the hydrogel; and F) the hydrogel is pushed back into the body of the stomach.
Floating drug delivery systems
Floating drug delivery systems have bulk density less than gastric fluids and so remain buoyant in the stomach without affecting gastric emptying rate for a prolonged period of time.12 While the system is floating on the gastric contents, the drug is released slowly at the desired rate from the system, after release of drug; the residual system is emptied from the stomach. This results in an increased gastric retention time and a better control of the fluctuations in plasma drug concentration. Floating drug delivery system can be divided into (i) Non-effervescent and (ii) Gas-generating system.13
Non-effervescent systems
This type of system, after swallowing, swells unrestrained via imbibitions of gastric fluid to an extent that it prevents their exit from the stomach. One of the formulation methods of such dosage forms involves the mixing of the drug with a gel, which swells in contact with gastric fluid after oral administration and maintains a relative integrity of shape and a bulk density of less than one within the outer gelatinous barrier. The air trapped by the swollen polymer confers buoyancy to these dosage forms.14 Excipients used most commonly in these systems include hydroxypropyl methyl cellulose (HPMC), polyacrylate polymers, polyvinyl acetate, Carbopol, agar, sodium alginate, calcium chloride, polyethylene oxide and polycarbonates. Various types of non-effervescent system are discussed below (a-c).
Such a system contains drug with gel-forming hydrocolloids meant to remain buoyant on the stomach content. This system incorporates a high level of one or more gel-forming highly soluble cellulose type hydrocolloid, e.g., hydroxypropyl cellulose, hydoxyethyl cellulose, hydroxypropyl methyl cellulose (HPMC), polysaccharides and matrix-forming polymer such as polycarbophil, polyacrylate and polystyrene. On coming in contact with gastric fluid, the hydrocolloid in the system hydrates and forms a colloid gel barrier around its surface. The schematic diagram of colloidal gel barrier system is shown in Figure 3.
This technology is based on the encapsulation of a drug reservoir inside a microporous compartment with pores along its top and bottom walls (Figure 4). In the stomach, the floatation chamber containing entrapped air causes the delivery system to float over the gastric content. Gastric fluid enters through the aperture, dissolves the drug and carries the dissolved drug for continuous transport across the intestine for absorption.
Multi-unit floating dosage forms have been developed from dried calcium alginate complex.15 Spherical beads of approximately 2.5mm in diameter can be prepared by dropping sodium alginate solution into aqueous solution of calcium chloride, causing the precipitation of calcium alginate. The beads are then separated, snap-frozen in liquid nitrogen, and freeze-dried at -40ºC for 24 hours, leading to the formation of a porous system, which can maintain a floating force for over 12 hours. Schematic diagram for preparation of alginate beads is shown in Figure 5.
Hollow microspheres/Microballons
Kawashima et al.16 was developed hollow microspheres loaded with drug in their outer polymer shell were prepared by a novel emulsion solvent diffusion method. The ethanol/dichloromethane solution of the drug and an enteric acrylic polymer was poured into an agitated solution of Poly Vinyl Alcohol (PVA) that was thermally controlled at 40ºC. The gas phase is generated in the dispersed polymer droplet by the evaporation of dichloromethane formed and internal cavity in the microsphere of the polymer with drug. The microballoon floated continuously over the surface of an acidic dissolution media containing surfactant for more than 12 hours. Various types of effervescent system are discussed below (a-c).
These buoyant systems utilize matrices prepared with swellable polymers such as methocel, polysaccharides (e.g., chitosan), effervescent components (e.g., sodium bicarbonate, citric acid or tartaric acid). The system is so prepared that upon arrival in the stomach; carbon dioxide is released, causing the formulation to float in the stomach.17,18 Other approaches and materials that have been reported are a mixture of sodium alginate and sodium bicarbonate, multiple unit floating pills that generate carbon dioxide when ingested, floating minicapsules with a core of sodium bicarbonate, lactose and polyvinylpyrrolidone coated with hydroxypropyl methylcellulose (HPMC), and floating systems based on ion exchange resin technology. These mini capsules contain a central core and a coating. The central core consists of a granule composed of sodium bicarbonate, lactose and a binder, which is coated with HPMC. Pepstatin is coated on the top of the HPMC layer. The system floats because of the CO2 release in gastric fluid and resides in the stomach for prolonged period.19
Sedimentation has been employed as a retention mechanism for pellets that are small enough to be retained in the folds of the stomach body near the pyloric region, which is the part of the organ with the lowest position in an upright posture.20 Dense pellets (approx. 3g/cm3) trapped in rugae also tend to withstand the peristaltic movements of the stomach wall. With pellets, the GI transit time can be extended from an average of 5.8–25 hours. Commonly used excipients are barium sulphate, zinc oxide, titanium dioxide and iron powder, etc. These materials increase density by up to1.5–2.4g/cm3.
Multiple unit type floating system is sustained release pills, known as ‘seeds’, which are surrounded by two layers. The outer layer is of swellable membrane layer and inner layer consists of effervescent agents. This system sinks at once and then it forms swollen pills like balloons which float as they have lower density, when it is immersed in the dissolution medium at body temperature. The lower density of the system is due to generation and entrapment of CO2 within the system.21
Ion exchange resins
A coated ion exchange resin bead formulation has been shown to have gastric retentive properties, which was loaded with bicarbonates. Ion exchange resins are loaded with bicarbonate and a negatively charged drug is bound to the resin. The resultant beads were then encapsulated in a semi-permeable membrane to overcome the rapid loss of carbon dioxide. Upon arrival in the acidic environment of the stomach, an exchange of chloride and bicarbonate ions take place. As a result of this reaction carbon dioxide was released and trapped in the membrane thereby carrying beads towards the top of gastric content and producing a floating layer of resin beads in contrast to the uncoated beads, which will sink quickly.
Osmotic regulated systems
It is comprised of an osmotic pressure-controlled drug delivery device and an inflatable floating support in a bio erodible capsule.22 The osmotic controlled drug delivery device consists of two components–drug reservoir compartment and osmotically active compartment. In the stomach the capsule quickly disintegrates to release the intragastric osmotically controlled drug delivery device. The inflatable support inside forms a deformable hollow polymeric bag that contains a liquid that gasifies at body temperature to inflate the bag.
Suitable drug candidates for gastro retention delivery system
It is evident from the recent scientific and patient literature that an increased interest in novel dosage forms that are retained in stomach for a prolonged and predictable period of time exists today in academic and industrial research groups. One of the most feasible approaches for achieving a prolonged and predictable drug delivery in the GI tract is to control the gastric residence time, i.e. gastro retentive delivery system.
Gastric retention is enhanced the therapeutic effect of the drugs due to improve the oral drug absorption in the stomach. Drugs are released from the formulations in controlled manner so that reduce dosing frequency and improve patience compliance. Suitable drug candidates for gastric retention delivery system are shown in (Table 1).
Poorly soluble at an alkaline pH |
|
Ranitidine |
Anti-Histamine |
Good absorption at stomach |
|
chlordiazepoxide |
Antipsychotic |
Cinnarizine |
Anti allergy |
Narrow absorption window |
|
Levodopa |
Ani epilepsy |
Riboflavin |
Vitamin |
Drug degradation at colon |
|
Ranitidine HCl |
Antiulcer |
Metronidazole |
Antimicrobial |
Amoxicillin |
Antibiotic |
Poor solubility in water |
|
Acyclovir |
Antiviral |
Silymarin |
|
Norfloxacin |
Antibiotic |
Ciprofloxacin |
Antibiotic |
Ofloxacin |
Antibiotic |
High solubility in acidic pH |
|
Dipyridamole |
Antiplatelet |
Domperidone |
Antiemetic |
Locally acting at stomach |
|
Misoprostol |
Anti Ulcer |
Table 1 Pharmaceutical and pharmacokinetics classification of drug candidates for gastro retentive delivery system
Application of gastric retention delivery systems on treatment of H. pylori infection
Helicobacter pylori (H. pylori) is one of the most common pathogenic bacterial infections, colonizing an estimated half the world’s population. It is associated with the development of serious gastro duodenal disease—including peptic ulcers, gastric lymphoma and acute chronic gastritis.23 Figure 6 is shown clear representation of mechanism of H. pylori induced gastric ulcer. H. pylori reside mainly in the gastric mucosa or at the interface between the mucous layer and the epithelial cells of the antral region of the stomach. H. pylori
Genomes have been linked to altered gastric acid secretion and premalignant histological features.
The discovery of this microorganism has revolutionized the diagnosis and treatment of peptic ulcer disease. Most antibacterial agents have low minimum inhibitory concentrations (MIC) against H. pylori in culture. And, single antibiotic therapy is not effective for the eradication of H. pylori infection in vivo. This is because of the low concentration of the antibiotic reaching the bacteria under the mucosa, instability of the drug in the low pH of gastric fluid and short residence time of the antibiotic in the stomach. Combination of more than one antibiotics and anti-secretory agent are required for complete eradication of H. pylori, but these regimens are not fully effective. Patient compliance, side effects and bacterial resistance are the other problems. Other than the multi-antibiotic therapy, different therapeutic strategies have been examined to completely eradicate H. pylori from the stomach.24
Drug delivery strategies for treatment of H. pylori
One way to improve the efficacy in eradicating the infection is to deliver the antibiotic locally in the stomach. Better stability and longer residence time will allow more of the antibiotic to penetrate through the gastric mucus layer to act on H. pylori. The reason for the incomplete eradication of H. pylori is probably due to short residence time of antimicrobial agents in the stomach so that effective antimicrobial concentration cannot be achieved in the gastric mucous layer or epithelial cell surfaces where H. pylori exists.25 The other reason may be the degradation of antibiotics in gastric acid. Access of antimicrobial drugs to the site is restricted from both the lumen of the stomach and the gastric blood supply. H. pylori may also have acquired resistance to the commonly used antimicrobial agents. As conventional drug delivery systems do not remain in the stomach for prolonged periods, they are unable to deliver the antibiotics to the site of infection in effective concentrations and in fully active forms. Therefore, it is necessary to design drug delivery systems that not only alleviate the shortcomings of conventional delivery vehicles but also deliver the antimicrobials to the infected cell lines. The absorption of an antibiotic into the mucus through the mucus layer (from the gastric lumen) is believed to be more effective for H. pylori eradication than absorption through the basolateral membrane (from blood). Scientists have focused on development of new drug delivery systems which were able to reside in stomach for an extended period for more effective H. pylori eradication.26–28
Polyelectrolyte coated multilayered liposomes (nanocapsules)
Jain P et al., was prepared polyelectrolylyte coated multilayered liposomes for complete eradication of H. pylori. The system possesses the advantages of both vesicular and particulate carriers, and it was prepared by alternative coating of polyanion (poly(acrylic acid), PAA) and polycation (poly(allylamine hydrochloride), PAH) using liposomes as the core. Compared with the conventional liposomes, the polyelectrolyte based multilayered system (nanocapsules) gave prolonged drug release in simulated gastric fluid, which is well suited for drug delivery against H. pylori infection in the stomach. In vitro growth inhibition study, agglutination assay, and in situ adherence assay in cultured H. pylori suggested the successful in vitro activity and binding propensity of the system. In vivo bacterial clearance study was carried out in a H. pylori infected mouse model. The novel system was found significant control of H. pylori infections.
Floating in situ gelling system
Gellan based amoxicillin and clarithromycin floating in situ gelling systems were prepared by dissolving varying concentrations of gellan gum in deionized water containing sodium citrate, to which varying concentrations of drug and calcium carbonate, as gas-forming agent.29,30 Required amount of amoxicillin and clarithromycin for eradication of H. pylori was less in this system than from the corresponding plain suspensions. Floating systems include gas-generating systems, non-effervescent systems have been used most commonly for treating H. pylori infections.31
Vaccine delivery systems (gastric-retention by mucoadhesive)
The most significant drawbacks of antibiotic therapy are its failure to prevent reinfection, and the increasing number of resistant strains; and these are the driving force to develop a vaccine against this infection.32 Mucosal vaccination offers protection against microorganisms which gain access to body via mucosal membranes. The advantages of mucosal vaccination are numerous and include high patient compliance, ease and low cost of application (i.e., no need of trained personnel) and a decrease in the risk of the unwanted needle-borne infections (AIDS, hepatitis, etc.). Further, vaccination at mucosal surfaces may stimulate both systemic and mucosal immunity; the latter not only at the site of vaccination, but also at distant mucosal epithelia.33,34 It could also prevent infection by neutralizing the pathogen at the site of entry.35 Chitosan based delivery systems are suitable for mucosal vaccination due to its ability to open up tight junctions and promote paracellular transport of antigen across mucosa.36,37
Nanoparticles
Concept of nanoparticulate muco-penetrating drug delivery system was developed complete eradication of Helicobacter pylori (H. pylori), colonized deep into the gastric mucosal lining. Due to nanoparticles has dual activity of adhesion and penetration of drugs into the mucous layer38 was developed pH-responsive chitosan/heparin nanoparticles by addition of heparin solution to a chitosan solution with magnetic stirring at room temperature. The nanoparticles appeared to have a particle size of 130–300nm, with a positive surface charge, and were stable at pH 1.2–2.5, allowing them to protect an incorporated drug from destructive gastric acids. Nanoparticles adhered to and infiltrate cell–cell junctions and interact locally resulting that significantly control H. pylori infections.39,40 Existing patented gastric retention drug delivery system suitable for treatment of H. pylori infection has outlined in the (Table 2).
Patent information |
Name of the delivery system |
Design of the drug delivery system |
US Patent Appl 2003232081. |
Floating bilayer tablet of |
Matrix forming gelling agent is HPMC which has a viscosity from 4000cps to 100000cps. Combination of matrix forming gelling agent of Methocels K4M and Methocels K100M. |
US Patent Appl 2006121106. |
SR gastroretentive amoxicillin composition in a |
The capsule may be a polymer material of HPMC, gelatin and starch. Preferably, HPMC. Suitable coatings may be well |
US Patent Appl |
CR matrix tablets, caplets, vegecaps, and capsules of one of the active agents from the group of Clarithromycin, |
Swellable polymers consist of one or more hydrophilic polymers such as guar gum, HPMC, CMC sodium salt, and xanthan gum. |
EP Patent 1416914. |
The Gastroretentive dosage form of a capsule containing the dried gel (film) with |
Combination of Xanthan gum and locust bean gum used for the preparation of film. sodium lauryl sulfate is used as Expansion agent. Viscosity adjuster is carbopol and polyvinyl pyrrolidone. Plasticizer used in films is polyethylene glycol. |
WO PCT Appln 02102415. |
CR buoyant dosage form consists of unit dose of Ofloxacin and Ciprofloxacin. |
Gel forming husk powder obtained from Lepidium sativum seeds, cross-linking |
Table 2 Patented gastric retention drug delivery systems used for treatment of H. pylori infection
Marketed products
Commonly used the formulation of gastroretentive dosage forms for treatment of H. pylori infection available in the market are listed in Table 3.
Name of the product |
Drug |
Design of the delivery system |
Cifran OD® |
Ciprofloxacin (1gm) |
Gas generating floating drug delivery system |
Cytotech® |
Misoprostol (100mcg/200mcg) |
Bilayer floating capsule technology |
Topalkan® |
Aluminum -magnesium antacid |
Effervescent floating liquid alginate preparation |
Amalgate Float |
Aluminum -magnesium antacid |
Floating dosage form |
Liquid Gaviscone® |
Aluminium hydroxide |
Colloidal gel preparation |
Table 3 Commercial product available in the market for treatment of H. pylori infection based on Gastric retention delivery systems
Gastro retentive drug delivery systems offers various potential advantages for drug with poor bioavailability due their absorption is restricted to the upper gastrointestinal tract (GIT) and they can be delivered efficiently thereby maximizing their absorption and enhancing absolute bioavailability. One of the main applications of gastric retention drug delivery system on treatment of H. pylori infection is promising area of research in pharmaceutical industry and academia. Based on literature, we concluded that gastric retention drug delivery system has more scope to file patent and lot of opportunity available to market the product which has more patient compliance.
None.
The author declares that there is no conflict of interest.

©2018 Gupta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.