Journal of
eISSN: 2473-0831


Case Report Volume 5 Issue 5
Correspondence: Gyan Chand, Department of Endocrine Surgery, Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, India, Tel -2494840, Fax -2669208
Received: June 30, 2017 | Published: July 28, 2017
Citation: Kumar A, Ranjan A, Mandal RP, Chand G, Rao RN (2017) Primary Neuroendocrine Tumor of Breast: Report of a Rare Case with Review of Literature. J Anal Pharm Res 5(5): 00154. DOI: 10.15406/japlr.2017.05.00154
The neuroendocrine tumors (NETs) are basically derived from the neuroendocrine cell which secrete different types of amines and peptides. The neuroendocrine cell is very rare in breast carcinoma of less than 0.1%. In the breast carcinoma, Primary neuroendocrine tumors are a heterogeneous series with 50% of cells having positive immunoreactivity to neuroendocrine markers. A case of 52 years woman with a lump in her right breast. She was evaluated for surgery and modified radical mastectomy (MRM) was done. Grossing of specimen showed two tumors in retro areolar and lower outer quadrant respectively. Histopathology revealed neuroendocrine tumor of the breast. All eighteen lymph nodes showed reactive lymphoid hyperplasia. Immunohistochemical report showed cells were positive for synaptophysin, chromogranin, neuron specific enolase (NSE) and Ki67 (60-70%). The estrogen receptor, progesterone receptor and HER-2Neu were negative. No mucin production was seen in this patient. Neuroendocrine tumors in the breast are mostly malignant. Mucinous carcinomas of breast have association with neuroendocrine differentiation but in this case although having neuroendocrine differentiation, is not associated with mucin production. Here we present a case of NET of the breast where tumor cells were non-reactive for ER (Estrogen Receptor), PRs (Progesterone Receptors) and cells show frequent mitotic figures. The review accompanying this case report is provide an overview of all the cases of primary neuroendocrine tumor of the breast published in the literature and detailed information regarding classification, epidemiology, clinical and histological diagnosis criteria, histogenesis, surgical and adjuvant treatment, as well as prognosis.
Keywords: neuroendocrine tumors, amine precursor uptake & decarboxylation, solid papillary carcinomas, mucinous carcinomas
MRM, modified radical mastectomy; NSE, neuron specific enolase; ER, estrogen receptor; PRs, progesterone receptors; NETs, neuroendocrine tumors; FNAC, fine needle aspiration cytology; MIBG, meta-iodobenzylguanidine
Neuroendocrine tumors (NETs) arise from the neuroendocrine cells and these cells are most commonly found in the gastrointestinal tract and respiratory system in our body. NETs of the breast are very rare and once they appear grow with slow rate. As per the previous available data, NETs of breast are contributing less than 1 % of all NETs and less than 0.1% for all breast cancer.1 The different histological types of breast carcinoma with focal ‘differentiated’ are found in situ and invasive ductal, lobular, colloid, papillary carcinoma, whereas the small-cell carcinomas are ‘undifferentiated’ kind of neuroendocrine breast carcinomas.2 The NETs is classified as primary and metastatic. In the Breast imaging study could not differentiated the primary from metastatic NETs.3 In clinical examination primarily focus on ruling out breast metastasis from small cell carcinoma of the lung, the gastrointestinal tract, pancreas, and the cervix. If the Metastatic NETs present in other organs cannot be distinguished from primary NETs because histologically they are similar,4 but Breast cancers with focal neuroendocrine differentiation typically have 12% to 18% of IHC-positive neuroendocrine cells.5 The incidence of primary NETs is range from 0.3% to 0.5%.2 The peer-reviewed articles reveal that the majority of cases have been described in female; the reported data suggest that NETs occurs between 20 to 83 years of age where the incidence is higher if the female patients aged ≥50 years.2 The tumors of other organs are uniformly positive for 50% Neuro Endocrine markers whereas the neuroendocrine tumors of breast carcinoma are observed as heterogeneous series.6 According to the previous studies, the Mucin production is a common feature in neuroendocrine breast carcinomas and it is correlated to a low aggressiveness of tumors.6 Neuroendocrine tumors in the breast are mostly malignant. Mucinous carcinomas of breast have association with neuroendocrine differentiation but in this case although having neuroendocrine differentiation, is not associated with mucin production. Here we present a case of NETs of the breast where tumor cells were non-reactive for ER (Estrogen Receptor), PRs (Progesterone Receptors) and cells show frequent mitotic figures. We also review the radiological diagnosis, clinical as well as pathological features of NETs and clinical management.
A case of 52 years woman presented at department of Endocrine Surgery OPD of Sanjay Gandhi Post Graduate Institute of Medical Sciences, Lucknow, with a lump in her right breast which was progressive for past one month. During clinical examination, there was two lumps were felt in upper outer quadrant of right breast. The larger one measuring about 4x4 cm and smaller one was 1.5X1.5cm, and lying larger above than smaller one. She had previous history of cholecystectomy and hysterectomy in 2002 and 2006 respectively. Fine needle aspiration cytology (FNAC) was done to take sample from that lump of right breast. The smears were analyzed under the microscopy, showing cohesive clusters and many dispersed atypical cells on hemorrhagic background and the reports revealed cells were positive for malignancy. She was evaluated for surgery and underwent right modified radical mastectomy (MRM) at our department. The specimen was sent for the further examinations. Grossing of specimen showed two tumors of dimension 3X3cm & 2X1cm in retro areolar and lower outer quadrant respectively. Histopathology revealed neuroendocrine tumor of the breast. All eighteen lymph nodes show reactive lymphoid hyperplasia. Immunohistochemical report showed cells were positive for synaptophysin, chromogranin, neuron specific enolase and Ki67 (60-70%). The estrogen receptor, progesterone receptor and HER-2Neu were negative. No mucin production was seen in this patient.
Neuroendocrine cells belong to APUD (Amine Precursor Uptake & Decarboxylation) system and secrete different types of amines and peptides. Neuroendocrine tumors can originate from various parts of the body and present with well-defined clinical entities, mostly in the gastrointestinal system. In previous studies, the knowledge about NETs has been demonstrated using histological and immunological technique but it has now possible to identify features of NETs morphological and biochemically. In 1963 several cases of invasive breast cancer seemed to have carcinoid growths which were formerly described by Feyrter et al.7 and further reconfirmed by Cubilla and Woodruff in 1977.7,8 At the end of the 1980s, chromogranin and synaptophysin were used as neuroendocrine differentiation markers, which were tested positive in argyrophilic breast carcinoma. A study was conducted in 1982, using both modified silver stain and electron microscopy were used to identify neurosecretory granules of NETs, suggestive of argyrophilic breast carcinoma in these patients.6 A specific definition for NETs was first proposed by Sapino et al in 2002, which was successively accepted by the World Health Organization (WHO) in 2003.9,10 These Morphological features of NETs are categorized by the WHO and these spindle cells arranged in round solid nests, plasmacytoid cells, signet-ring shaped mucinous cells and immunohistochemically positivity (˃50% of the tumor cell population) for the neuroendocrine markers such as synaptophysin and Chromogranin.2,10 In 2012, as per revised classifications of NETs are confirmed by WHO as a well-differentiated, poorly differentiated, and invasive breast carcinoma with NE differentiation. The diagnosing of NETs in breast also requires fulfilling two other criteria such as, the tumor must show histological evidence of a breast in situ component and other primary sites must be ruled out.2,11 The histogenesis of NETs has still controversial. An early theory suggested that NETs cells or argyrophilic cells are derived from neural crest cells that migrated to the mammary ducts8 whereas results from an early divergent differentiation event in breast carcinogenesis in which neoplastic stem cells differentiate into both epithelial and endocrine lines.2 Primary NETs present in the breast generally found as an intraductal lesion, which is not associated with any definitive gross pathological characteristics. But it has presence of histopathological features including dilated ducts filled with ovoid, polygonal, and spindle shapes cells and a low- or moderate- grade of nuclear atypia. The intraductal element of NETs may be mistaken for either atypical intraductal hyperplasia or atypical papilloma.10,12 The production of mucin is highly significant because the solid papillary/ mucinous carcinomas produce longer survival times than other subtypes of NETs.6
Diagnosis
It is difficult to diagnose NETs of the breast because the patients with primary NETs do not have any distinctive clinical presentation. If the tumors sizes are <2.0cm, patients are staged with early breast cancer.2 The radiological procedure such as mammography and ultrasonography, there was evidence of a highly-dense mass with a speculated or micro lobulated margin in mammography and a homogenously hypoechoic massive lesion in ultrasonography.2 On mammogram these tumors may lack the typical features of carcinoma breast like micro- calcification, irregular margins and so may be labeled as benign lesion. FNA or core needle biopsy examination is necessary for the diagnosis.
Immunohistochemistry
NETs exhibit different types of neuroendocrine markers like chromogranin A, synaptophysin and neuron-specific enolase. Among these markers, chromogranin A is the most specific marker for neuroendocrine breast carcinoma. The Diagnostic criteria for any NETs require that 50% of the cells should be neuroendocrine marker positive. In the present case study it was positive immunoreactivity for Chromogranin (Figure 1), synaptophysin (Figure 2), Neuron specific enolase (Figure 3) and Ki67 (Figure 4). As in our case all these markers are positive. NEBC (Neuro Endocrine Breast Carcinoma) are more likely to be ER/PR positive and HER-2 negative.13,14 However, in our case tumor cells is non-reactive for estrogen and progesterone receptors (Figure 5).
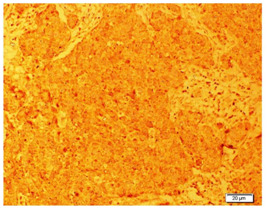
Figure 1 Photomicrograph showing immunohistochemical localization of chromogranin a in breast tissue. It is noted that tumor cells were positive with the chromogranin a marker.
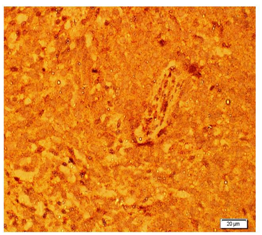
Figure 2 Photomicrograph showing immunoreactivity of Synaptophysin neuroendocrine marker in breast tissue.
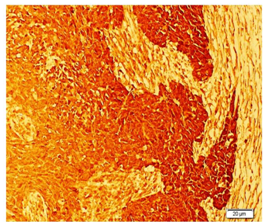
Figure 3 Photomicrograph showing positive immunohistochemical localization of Neuron specific Enolase (NSE) in breast tissue.
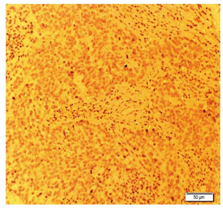
Figure 5 Imunohistochemical photomicrograph showing positive localization of estrogen marker in breast tissue.
Histopathology
In the Histopathological examination of breast NETs resembles with lung and gastrointestinal NETs.2 According to the WHO, there are three histologic categories of breast NETs (solid carcinoid-like, large cell-type, and small cell-type).10 The growth of cells should be in solid sheets or insular pattern, presence of stippled chromatin and low grade cytological features.13 A histopathological examination of our patient made us consider the presence of neuroendocrine differentiation because the sections of breast nodule shows infiltrating tumor arranged in sheets and nests of atypical cells separated by fibrous bands and round to oval vesicular nuclei with powdery chromatin was seen. Further, the cytoplasm was scanty and frequent mitotic figures were present (Figure 6).
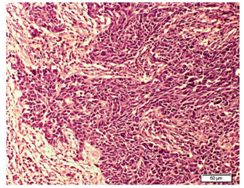
Figure 6 Photomicrograph of H&E stained section showing well stained tumor cells were present in breast tissue surrounding connective tissue.
Treatment
As NETs of breast are rare and very few cases had been reported, so standard therapy remains controversial. Surgery is still important in the management of breast NETs according to the stage of disease (lumpectomy with sentinel node biopsy to modified radical mastectomy). The current recommendation for primary NETs patients to give thehormone therapy is based ontheir hormonal receptor status (immunohistochemically receptor positive for ER 80% PR 35%). Due to the presence of specific cellular receptors in NETs of the breast, somatostatin and interferon has been claimed as a useful tool both for diagnosis and therapy. The chemotherapy regimens reported in the previous literature include fluorouracil/ epirubicin/ cyclophosphamide followed by docetaxel, etoposimide and carboplatin or cisplatin; cisplatin/ irinotecan, Adriamycin and cytoxan or cisplatin, paclitaxel alone, and cyclophosphamide/ methotrexate/fluorouracil.2 As for therapy, radio-labelled synthetic analogs of somatostatin show advantages as compared to native somatostatin because of longer half-life in cases that had positive octereotidescan.15 In neuroendocrine cells of breast,the meta-iodobenzylguanidine (MIBG) isabsorbed and concentrated so thattherapeutic use of MIBG has been tested in metastatic cases.16
Prognosis
A prognosis is difficult to make owing to the lack of long-term survival data among such patients. As in breast carcinoma of the usual type, size is an important prognostic factor in this tumor. The stage of the disease at the time of diagnosis is a determinant factor in its evolution.17 ER and PR are important markers for directing therapy and determining prognosis. Low grade NETS with low proliferation rate and ER expression are to be considered to have a better prognosis.18,19 Histologic grade is the most important predictor of prognosis. Solid neuroendocrine tumor is considered to be well-differentiated tumors. However, small cell and large cell neuroendocrine tumor are poorly differentiated. Hence, we can assert that patients with a solid neuroendocrine tumor or have a better prognosis than those with small cell and with large cell neuroendocrine tumor. Two prognostic factors identified in a retrospective study are Regional lymph node metastasis and high nuclear grade.15
In summary, this study showed that NETs in the breast are mostly malignant. Mucinous carcinomas of breast have association with neuroendocrine differentiation. In this case although having neuroendocrine differentiation, but it is not associated with mucin production. The production of mucin is highly significant because the solid papillary/ mucinous carcinomas produce longer survival times than other subtypes of NETs.3 The radiological procedure are giving evidence of a highly-dense mass with a speculated or micro lobulated margin in mammography and a homogenously hypoechoic massive lesion in ultrasonography. Surgery is still important in the management of breast NETs according to the stage of disease (lumpectomy with sentinel node biopsy to modified radical mastectomy). The ER and PR are important markers for directing therapy and also determining prognosis. The current recommendation for primary NETs patients to give the hormone therapy is based on their hormonal receptor status (immuno-histo-chemically receptor positive for ER 80% PR 35%). Being aware of the existence of NETs may allow for timely diagnosis.
Corresponding author acknowledge department of endocrine surgery for performing clinical activities and support from other department for valuable outputs.
The authors declare that there are no conflicts of interest.

©2017 Kumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.