Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 4
Correspondence: Yoshihiro Kudo, Graduate School of Science, Chiba University, 1-33 Yayoi-cho, Inage-ku, Chiba 263-8522, Japan, Tel -3038
Received: June 05, 2017 | Published: July 18, 2018
Citation: Kudo Y, Suzuki Y. Potentiometric study of liquid membrane transports of ionic Na(I) species with some crown ethers into nitrobenzene and 1,2-dichloroethane. J Anal Pharm Res. 2018;7(4):375-381. DOI: 10.15406/japlr.2018.07.00254
Potentiometric measurements of the following liquid-membrane-transport cell were performed at 298 K. Cell: (-) Ag|AgCl | 0.05mol dm−3 (C5H11)4N+Cl- ¦ nitrobenzene (NB) or 1,2-dichloroethane (DCE) ¦* 0.05mol dm−3 NaCl with L, NaOH, and picric acid | AgCl|Ag (+), where the symbols ¦ and L denote an interface with a sintered glass and a crown ether, respectively. 15-Crown-5 ether (15C5), benzo-15C5, 18-crown-6 one (18C6), and benzo-18C6 were used as L. Assuming emf={distribution equilibrium potentials (Δϕeq/V) around the NB/- or DCE/water interface expressed by the asterisk} + other potentials in the cell, the relation of (F/RT)emf » kt + ln KD¢ was derived under the conditions of KD>>1 and a steady state. Here, emf, k, t, KD and KD¢ refer to an emf (mV) measured between the two Ag/AgCl electrodes, an apparent first-order-reaction rate constant (minute−1) for the distribution of Na(I) species, a monitored time (minute), a conditional distribution constant of the Na(I) species into the NB or DCE phase, and its modified KD, respectively. With the Δϕeq values previously-reported at 298K, positive correlations of the ln KD¢ values obtained from the (F/RT)emf-vs.-t plots were observed in the systems with the four L employed; their correlation coefficients obtained from Δϕeq-vs.-ln KD¢ plots were 0.909 for the NB system and 0.896 for DCE. These good correlations indirectly confirmed the presence of Δϕeq for the liquid-membrane transport systems. The emf values of another cell was also analyzed.
Keywords: potentiometry, liquid-membrane transports, distribution equilibrium-potential, crown ethers, nitrobenzene
There are distribution equilibrium potentials (dep or Δϕeq) for single ion transfers across liquid/liquid interfaces1–3 in the field of electrochemistry and then it has been reported that these dep values between bulk organic (org) and water phases are present in univalent- and divalent-cation extraction systems without or with crown ethers (L).4–12 Also, in these studies, the term of “a conditional distribution constant” has been used for distinguishing the distribution constant of the ion with the dep ≠ 0 V from that of neutral species with usually dep=0V.10–12 However, in the present state, such studies have been limited to the narrow field, such as the above extraction phenomena.4–12 This fact can cause the supposition that the presence of dep in the extraction systems is a rare case. On the other hand, as an experiment similar to the extraction ones, the experiments of liquid-membrane transport or separation of cations (Mz+) with various L have been reported so far at Mz+=Na+, K+, Cs+, Sr2+, Cu2+, Cd2+, Pb2+,13–18 protonated D- and L-Phe,19 and so on.20
In the present paper, in order to clarify the dep presence based on an experiment other than the extraction and electrochemical ones, we tried liquid-membrane transport experiments of Na+ with L into nitrobenzene (NB) and 1,2-dichloroethane (DCE) at 298K by using potentiometry. In such potentiometric measurements, handmade Ag/AgCl electrodes were immersed in the so called source phase of the Na(I) species, such as Na+ and NaL+, with Cl−, OH−, and picric acid (HPic) and their receiving phase with (C5H9)4N+Cl− (TBA+Cl−) and thereby an emf value between these two electrodes was monitored during the ion transport measurements with the liquid membrane of NB or DCE. As L11, 15-crown-5 ether (15C5), benzo-15C5 (B15C5), 18-crown-6 ether (18C6), and benzo-18C6 (B18C6) were employed. Also, theoretical relations were derived for the analyses of the emf-versus-time (t) plots of two kinds of cells (see the emf measurements section). From these analyses, apparent rate constants, such as k and k¢, and the conditional distribution constants and other ones, such as KD¢ and KNa/NaL¢, for the membrane transport of Na+ with L were obtained. So, the plots of Δϕeq versus ln KD¢ for the NB and DCE systems with the four L employed gave good linear correlations. Besides, the speed of the process NaL+org→NaL+ was compared with those of the processes, NaL+→NaL+org and NaL+org→Na+ +Lorg.
Materials
Sodium chloride {guaranteed reagent (GR): >99.5%, Kanto Chemical, Tokyo} was dried at >100°C for more than 8h. Tetrabutylammonium chloride (>98.0%, Tokyo Chemical Industry) and NaOH (extra pure: >93.0%, Wako Pure Chemical Industries, Tokyo) were used without further purifications. An aqueous solution of HPic×xH2O (GR: >99.5%, added water: 15-25%, Wako) was prepared with pure water and its concentration was determined by the acid-base titration with phenolphthalein. Benzo-15C5 and B18C6 were purchased from Tokyo Chemical Industry and the former was dried in vacuo at a room temperature for 10h. 15-Crown-5 ether and 18C6 were purchased from Kanto Chemical and Acros Organics, respectively. The melting points of these ethers except for 15C5 were measured. When the observed ranges of their points were about one degree C, the ethers were used without any purification. Nitrobenzene (GR: >99.5%, Kanto & Wako) and DCE (GR: >99.5%, Kanto) were washed three times with pure water and stored in states saturated with water.5,11 Other chemicals were of GR grades. Pure water was prepared with the same procedure as that reported before.10,11
Emf measurements
The emf measurements were performed with Cells (A) and (B) at T=298K and P=1013h Pa.
Cell (A): (-) Ag|AgCl | 0.05mol dm−3 TBA+Cl− (phase I) ¦
NB or DCE (II) ¦* 0.05mol dm−3 NaCl with L, NaOH, and HPic (III) | AgCl|Ag (+).
Here, a sintered glass, depicted by ¦ in the cells, separated the phase I from the phase II and also did the phase III from the phase II. Aqueous solutions of NaCl in the phase III and TBA+Cl- in the I were prepared at the concentrations of 0.050mol dm-3 and 0.051, respectively. In the cell (A) of the NB/water systems, the concentrations of L, HPic, and NaOH were in the ranges of (1.7-4.2)×10-5mol dm-3 for the four L and additionally 0.0031 for B15C5, (2.3 or 4.1)×10-4 and (3.0 or 5.0)×10-4, respectively.11 Also, in the DCE/water systems, those of L, HPic, and NaOH were in the range or concentrations of (1.6-3.0)×10-3mol dm-3, 0.0026, and 0.0026, respectively.11
Cell (B): (-) Ag|AgCl | 0.05mol dm−3 TBA+Cl− (phase I) ¦
L=B15C5 or B18C6 in NB or DCE (II) ¦* 0.05mol dm−3 NaCl with NaOH, and HPic (III) | AgCl|Ag (+).
In this cell, the concentrations, CL,org, of L=B18C6 in the NB (=org) phase, B15C5, and B18C6 in the DCE ones were 3.01×10-5mol dm-3, 0.00165, and 0.00206, respectively, and those of the materials in the other phases were the same as the concentrations in the cell (A), except for the absence of L in the phase III. Silver/silver chloride electrodes were of handmade in a common procedure in which the aqueous solutions of 1% K[Ag(CN)2] (GR: >99%, Kanto) and 0.1mol dm−3 NaCl were used for their electrolytic preparation. The potentials of the handmade Ag/AgCl electrodes were regularly checked with the following cell: (-) Ag|AgCl | 0.1mol dm−3 NaCl | Ag|AgCl (+).
From the TBA+Cl- in the phase I (about 3cm3) contacted with the Ag/AgCl electrode, the mixture (about 3cm3) of NaCl, L, HPic, and NaOH in the III with the electrode were separated by the phase II of NB or DCE. Here, the phases I and III with Ag/AgCl electrodes were sealed up in glass containers of about 3cm3; their containers have been used in a cell for the ion-transfer polarographic measurements.21 A U-type-like cell was made between the org phase II (about 40cm3) in a 100cm3 beaker and the two containers put up perpendicularly. The phase II prepared in the beaker was magnetically stirred with a Teflon-coated stainless bar at a constant speed during the emf monitoring. After a setting of the cell (A) or (B), the monitoring time t was measured from a start of the agitation of the org phase II. The pH/ion meter (TOA, type IM-20E) was used for the emf measurements (within ±1mV) of the cells, in which the phase II was kept at 25±0.4°C as priority. The same measurements with the other meter (Horiba, type F23) were not able to indicate stable emf readings, though their reasons are unclear.
Theoretical handling
A theoretical handling of the measured emf values based on the membrane transport models13,14,17 of the ion with L is described as follows. We monitored an emf value of the following bulk-liquid-membrane cell:
Cell (A): (-) Ag|AgCl | 0.05mol dm−3 TBA+Cl− (phase I) ¦
NB or DCE (II) ¦* 0.05mol dm−3 NaCl with L, NaOH, and HPic (III) | AgCl|Ag (+).
Using inner potentials of the five phases, its emf value measured can be expressed as
Emf=(ϕAg/AgClright − ϕIII) + (ϕIII − ϕII) + (ϕII − ϕI) + (ϕI − ϕAg/AgClleft) (1)
= (ϕAg/AgClright − ϕIII) + (ϕIII − ϕII) + (ϕII − ϕI) − (ϕAg/AgClleft − ϕI),
where the symbol ϕq denotes the inner potential of the phase q (=Ag/AgCl, I, II, and III). Actually Eq. (1) becomes
Emf = ϕAg/AgClright − ϕAg/AgClleft » (ϕIII − ϕII) + (ϕII − ϕI) (1a)
with (ϕAg/AgClright – ϕIII ) - (ϕAg/AgClleft − ϕI) » 0V, because of the presence of Cl− being equivalent in the phases I and III.
Also, considering a j-ion transfer between the bulk phases II and III with the interface expressed by the asterisk, the following process can be proposed:1–12
Then, assuming that this process is simply of the first order reaction13,15 (the assumption 1) and accordingly its forward rate constant (k) is larger than the corresponding backward constant (kb) (the assumption 2), we can employ the general relation:13,15,22
C = C0exp (−kt). (2a)
Here, C and C0 refer to the concentration of the ion j at the time t and an initial one of j in the phase III, respectively. Moreover, using the mass balance equation, C0=C+Corg (=CIII+CII), at t and rearranging Eq. (2a), we can immediately obtain
KD+1=exp(kt) (2b)
with KD=Corg/C. If the conditional distribution constant KD is much larger than unity (the assumption 3), then we can simplify Eq. (2b) as
KD » exp (kt). (3)
According to our previous papers10–12 the KD value can be related to the Δϕeq or dep as follows:
Δϕeq=(RT/zjF)(ln KD,j−ln KD,jS). (4)
Here, zj shows a formal charge of j with the sign and KD.jS does a standard distribution constant of j into the org phase, namely KD,j at Δϕeq=0 V.10–12 Rearranging Eq. (1a) based on Eq. (4) gives the following equation.
Emf » (Δϕeq,j+ − Δϕeq,j− + ΔELJ + iRs) + (ϕII − ϕI) = Δϕeq,j+ + α
» (RT/zj+F)(ln KD,j+ − ln KD,j+S) + α (1b)
with α=ΔELJ+iRs−Δϕeq,j−+(ϕII−ϕI), where ΔELJ and iRs denote a liquid junction potential between the phases II and III23 and an ohmic potential drop24 in the cell, respectively, and this potential α was considered to be a constant within an experimental error. At equilibrium Δϕeq,j+ equals |Δϕeq,j−|, while at the steady state (the assumption 4, see below) Δϕeq,j+ can be a little different from |Δϕeq,j−|. Introducing Eq. (3) in Eq. (1b) and then rearranging it, we can immediately obtain
(zF/RT)emf » kt+ln KD¢. (5)
with ln KD¢=(zF/RT)α−ln KDS. (5a)
Here, the subscripts, j and j+, were omitted for visual simplicity of Eqs. (5) and (5a). Therefore, we can obtain the k and ln KD¢ values from the plot of (zF/RT)emf versus t (Figure 1). In Eq. (5), we can think that the KD¢ term is a conditional distribution constant for j with Δϕeq≠0V for the membrane transport system, as well as the NaPic extraction ones with L.11 One can also suppose that the ln KD¢ and (F/RT)α terms of Eq. (5a) correspond to the (F/RT)Δϕeq and ln KD,j ones, respectively, at z=zj=1 in comparison with Eq. (4). Moreover, considering the cell constitution, it is valid that the k term means the apparent first-order-reaction rate constant for the ion transfers from the bulk phase III to the II.
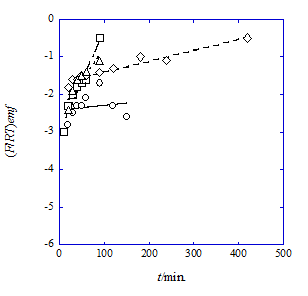
Figure 1 Plots of (F/RT)emf vs. t for the NB system with 15C5 (circle), B15C5 (square), 18C6 (diamond), and B18C6 (triangle) at T=298K. The correlation coefficients (R) were 0.211 for 15C5, 0.968 for B15C5, 0.972 for 18C6, and 0.923 for B18C6.
Similarly, the cell (B) was employed for the emf monitoring of only the systems with the benzo derivatives in the org phase. The constitution of this cell comes from the distribution properties, KD,L>>1, of L=B15C5 and B18C6, where KD,L is similarly called the distribution constant and defined as [L]org/[L].25 In this case, the ion transfer process is mainly
with KNa/NaL=[NaL+]org/[Na+][L]org. (6a)
As the rate law prepared for Eq. (6), we can derive
−d[Na+]/dt =−d[L]org/dt=d[NaL+]org/dt=k1[Na+][L]org−k2[NaL+]org, (7)
where the symbols ki denote the rate constants at i=1 or 2. Commonly, k1 corresponds to the rate constant of the process Na+ +Lorg→NaL+org, while k2 does to that of NaL+org→Na+ +Lorg. Introducing Eq. (6a) in Eq. (7) and rearranging it, the following equation is obtained:
−d[Na+]/dt=[L]org(k1−k2KNa/NaL)[Na+]=k¢[Na+] (8)
with k¢/[L]org=k1−k2KNa/NaL (> 0). (8a)
So, Eq. (8) can express the modified rate law for the process Na+ +Lorg→NaL+org and Eq. (8a) corresponds to a pseudo-second-order-reaction rate-constant. Comparing Eq. (8) with the rate law, −d[j]/dt=k[j], of Eq. (2), we can see easily that Eq. (2a) is useful for the analysis of the emf data obtained from the cell (B) too.
From Eqs. (2a) and (2b), we can obtain C=C0exp (−k¢t) and KNa/NaLCL,org+1=exp (k¢t), respectively, with the mass balance equation, C0=C+Corg=[Na+]III +CII. Then, rearranging the latter equation with the term KNa/NaLCL,org, we can easily derive KNa/NaLCL,org=exp (k¢t){1- exp (-k¢t)}. By taking a natural logarithm of the both sides of this equation, the following equation can be given: ln KNa/NaL=k¢t+ln Γ-ln CL,org with Γ=1-exp (-k¢t). Consequently it becomes
ln KNa/NaL=k¢t+ln Γ−ln CL,org ≈ ln KD+ln KNaL,org (3a)
with KNaL,org=[NaL+]org/[Na+]org[L]org. So, from Eqs. (1b) and (3a), we can easily obtain
(F/RT)emf + ln CL,org»k¢t+ln KNa/NaL¢×Γ. (9)
with ln KNa/NaL¢=(F/RT)αB−ln KDSKNaL,org (9a)
at z=1 and α=αB under the assumption of ln Γ=constant value (the assumption 3b). Using Eq. (9) and assuming that CL,org equals a total concentration of L in the org phase (see the emf measurements section), a plot of (F/RT)emf + ln CL,org versus t yields k¢ as the slope and ln KNa/NaL¢× Γ as the intercept (Figure 2). Also, Eq. (9a) is similar to Eq. (5a) in its equation form.
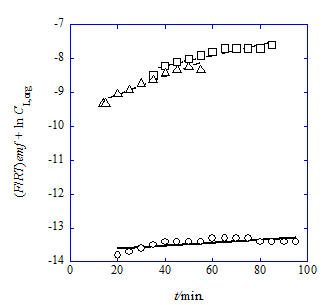
Figure 2 Plots of (F/RT)emf + ln CL,org vs. t for the NB system with L=B18C6 (circle) and the DCE ones with B15C5 (square) and B18C6 (triangle) of the cell (B) at T=298K: see the text for CL,org. The regression lines were based on Eq. (9).
For the assumptions employed in the above handling, the assumptions 1 to 3 and 3b were essentially satisfied with the above experiments with emf: see the following results and discussion sections. On the other hand, the validity of the assumption 4 may be shown in the fact that the KD¢ values hardly reflect the KD,NaL and KD,Pic ones. Strictly speaking, it is difficult to clarify whether the assumption 4 is satisfied or not.
The following results were obtained from only the measurements of emf with t. Figure 3 & Figure 4 show emf-versus-t plots of Cell (A) (see the emf measurements section) at 298K for the NaCl/HPic-15C5 system with NB and -18C6 one with DCE, respectively. Here, the symbol t means the monitoring time in min. of emf. As shown in these figures, the NB systems were superior to the DCE ones in the stability of the emf measurements. Average values of the data sets of 3 to 5 for a given t were used for the analyses of the (zF/RT)emf-versus-t plots based on Eq. (5) (see the theoretical handling section) at z=1. In order to bring the data sets close to the equilibrium condition, considering the steady state condition of the system (the assumption 4, see the theoretical handling section), the emf values over t=20 or 10 minutes were employed for the data analyses; the triangle data in Figure 4 were neglected because of their less stability.
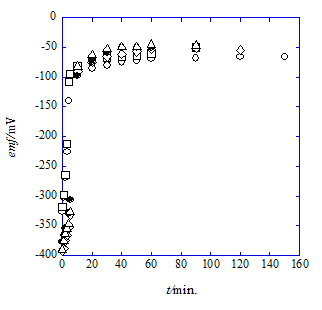
Figure 3 Plots of emf vs. t for the NaCl/HPic-15C5 system with NB of the cell (A) (see the theoretical handling section) at C15C5=4.22´10−5mol dm-3.
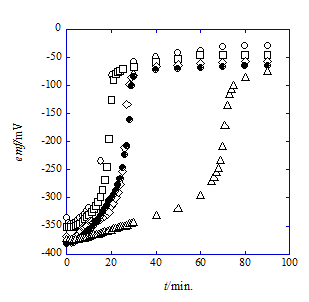
Figure 4 Plots of emf vs. t for the NaCl/HPic-18C6 system with DCE of the cell (A) at C18C6=0.00303mol dm-3.
Figure 1 shows the (F/RT)emf-versus-t plots for the NB systems. As an example, the regression line of L=15C5 was (F/RT)emf = (0.0016 ± 0.0030)t + (−2.44 ± 0.24) {See Eq. (5)} at R=0.211. This less R value should be caused by the value of the slope being close to apparently zero and its larger deviation (Figure 1). Similar plots were observed in the other NB and DCE systems; for the latter system, the R values were in the range of 0.84 to 0.98. Except for the 15C5 system with NB, the other systems virtually yielded linear equations, suggesting that the assumption 1 (see the theoretical handling section) holds. Table 1 summarizes the k and ln KD¢ values determined here. Also, the half-life (τ1/2) values were estimated from the k ones22 and listed in Table 1. In the τ1/2 values of the NB system, 15C5 and 18C6 were much larger than the other L.
L |
k a/min.-1 (τ1/2b/min.) |
ln KD¢ |
Δϕeqc/V (log KD,NaLc) |
|||
org=NB |
DCE |
NB |
DCE |
NB |
DCE |
|
15C5 |
0.0016±0.0030(4.3×102) |
0.025±0.005(28) |
−2.44±0.24 |
−4.12±0.25 |
0.12(2.03) |
0.11(0.4) |
B15C5 |
0.027 ±0.003(26), |
0.019±0.004(36) |
−2.99±0.15, |
−3.70±0.80 |
0.024(2.61) |
0.14(1.8) |
18C6 |
0.0029±0.0002(2.4×102) |
0.044±0.009(16) |
−1.68±0.04 |
−5.72±0.61 |
0.15(2.28) |
0.022(0.84) |
B18C6 |
0.018±0.004(39) |
0.036±0.009(19) |
−2.51±0.20 |
−3.92±0.56 |
0.098(4.24) |
0.078 (4.57) |
Table 1 Fundamental data obtained from the analyses of the liquid-membrane transport cell (A) with NaCl/HPic and some L at 298K
aForward rate constant of the cell (A) in the text. bThe half life calculated from τ1/2=ln 2/k. See ref. 22.
cData determined by extraction experiments in ref. 11. dExperimental values to the initial concentration of 0.00310mol dm−3 B15C5.
Figure 5 shows the emf-versus-t plots at 298K for Cell (B) (see the emf measurements section) of the NaCl/HPic-B18C6 system with NB and the NaCl/HPic-B15C5 and -B18C6 ones with DCE. The monitoring t values which are more than 20min. for the B18C6 system with NB, 35 for B15C5 with DCE, and 14 for B18C6 with DCE were employed for the following analyses and the emf values of three data sets were averaged for a given t. The plots of (F/RT)emf + ln CL,org {=F(B)} versus t (Figure 2) were analyzed by Eq. (9). The results were listed in Table 2.
L |
k 'a/min.->1 (τ1/2b/min.) |
ln KNa/NaL'-Γ |
||
org=NB |
DCE |
NB |
DCE |
|
B15C5 |
---c |
CL,DCEd=0.00165 |
---c |
−8.78±0.13 |
B18C6 |
CL,NBd=3.01´10−5 |
CL,DCEd=0.00206 |
−13.69±0.06 |
−9.59±0.09 |
Table 2 Fundamental data obtained from the analyses of the liquid-membrane transport cell (B) with NaCl/HPic and some L at 298K
aForward rate constant of the cell (B) in the text. bThe half life calculated from τ1/2=ln 2/k. See ref. 22.
cNot determined. dExperimental values/mol dm−3.
The plots of dep versus ln KD¢ for the both systems are shown in Figure 6. These regression lines were Δϕeq=(0.10±0.03) ln KD¢+(0.34±0.07) at R=0.909 for the NB system and Δϕeq=(0.049±0.017) ln KD¢+(0.30±0.08) at 0.896 for DCE.
Characterization of the k and ln KD¢ values
In the NB systems, the k values of the benzo derivatives were by one order larger than those of the simple ethers (Table 1). On the other hand, in the DCE systems, there were no large differences in k among the L employed. Or, it seems that the k values of the 18C6 derivatives are larger than those of the 15C5 ones among the DCE systems. These values for the 15C5 and 18C6 with NB (Table 1) were close to the observed first-order rate constants (=0.0039-0.0079min.−1) at 298K for K+ complexes of the carboxylic 18C6 derivative with CHCl3,14 while those for all the L with DCE were comparable to the rate constants (=0.047 & 0.056min.−1) observed at 298K for the Na+ complexes of B15C5 and B18C6 with CH2Cl2.13 These agreements indicate the validity of the present method described in the theoretical handling section. Also, it is interesting that the rate constants13,14 reported for the transfer of all species, such as M+, ML+, and MLA (neutral ion-pair complex with a pairing anion A-), are comparable to those evaluated here. This suggests that the major transport species in the membrane transport experiments are ionic, not neutral ones.
According to our previous paper,11 the log KD,NaL values (Table 1) were in the orders L=15C5<18C6<B15C5<B18C6 for both the NB and DCE systems. Also, the log KD,Pic values were in the orders 18C6 (log KD,Pic=−2.5) <15C5 (−2.0) <B18C6 (−1.6) <B15C5 (−0.4) for NB and B15C5 (−3.3)<15C5(−2.90)<B18C6(−2.32)<18C6(−1.38) for DCE.11 On the other hand, the ln KD¢ values in Table 1 were in the orders B15C5<B18C6»15C5<18C6 for NB and 18C6<15C5£B18C6£B15C5 for DCE. The NB systems show the tendency of hydrophobic L < hydrophilic L, but the DCE ones do its reverse tendency. The above orders indicate that the KD,NaL and KD,Pic values “at equilibrium” hardly reflect the KD¢ ones “at the steady state”. Being different from the KD,Pic and KD,Na (<KD,Pic) values,11 however, only the KD,NaL values satisfy the assumption 3 described in the theoretical handling section. The charge balance equation, [Na+]org+[NaL+]org=[Pic-]org, in the extraction experiments11 shows the distribution of Na+, NaL+ and Pic- (especially NaL+ & Pic-) into the org phase, in addition to the distribution of the charge-less NaLPic. The log KD,NaL values have revealed the relations of org =NB>DCE for all the L (Table 1). The same is true of the relation, NB > DCE, of the log KD,Pic values,11 except for 18C6. The ln KD¢ values showed the same relations, except for the case of NB<DCE for L=B18C6. These results suggest that a strength of the ion-solvent interaction controls a magnitude of ln KD¢. On the basis of the above results, the KD¢ values seem to reflect a comprehensive distribution of NaL+ and Pic−.
Indirect evidence for the presence of dep
The dep or Δϕeq order listed in Table 1 was B15C5<B18C6<15C5<18C6 for NB and 18C6<B18C6< 15C5 <B15C5 for DCE. These orders are close to those of the ln KD¢ values (see above). Additionally, as described in the theoretical section, we can see the possibility that the (F/RT)Δϕeq term is comparable to the ln KD¢ one: see Eqs. (4) and (5a) (see the theoretical handling section). Figure 6 indirectly prove the presence of the dep between the two bulk phases II and III with the NB/- or DCE/water interface. Also, the results indicate that there are the dep values in the liquid membrane transport, as well as solvent extraction and electrochemistry at liquid/liquid interfaces. Moreover, the intercepts show the Δϕeq values for the hypothetical L system which satisfies the condition of ln KD¢=0, namely α=(RT/F)ln KDS in Eq. (5a). In other words, this makes us suppose α is a constant under the constant condition of P and T.
Determination of the ln KNa/NaL¢ and k2¢ values
The authors examined the assumption 3b (see the theoretical handling section) using the definition of ln Γ [= ln {1−exp (−k¢t)}] with the above experimental k¢ (Table 2) and t values. The calculated ln Γ values were −1.57±0.41 for the B18C6 with NB in the t range of 20-95min., −0.58 ± 0.18 for B15C5 with DCE in that of 35-85 and −0.63±0.31 for B18C6 with DCE in that of 14-55. On the basis of these values, the corresponding ln KNa/NaL¢ values were estimated to be −12.12 ± 0.41, −8.20 ± 0.22, and −8.96 ± 0.32, respectively. Such experimental errors of ln Γ and ln KNa/NaL¢ for L=benzo derivatives were comparable to those of ln KD¢ in Table 1. The ln KNa/NaL¢× Γ value (=the intercept, Table 2, Figure 2 & the theoretical handling section) for NB was somewhat smaller than the ln KNa/NaL¢ one, while the intercepts for DCE were close to the ln KNa/NaL¢ values. These results essentially satisfy the assumption 3b for the present case and indicate that the intercepts of the F(B)-versus-t plots are adequately close to the ln KNa/NaL¢ values.
Also, the log KNa/NaL values were calculated to be 3.48 for L=B18C6 with NB, 0.3 for B15C5 with DCE, and 1.27 for B18C6 with DCE from the thermodynamic relation,12 KNa/NaL=KD,NaKNaL,org. From the relation26 at equilibrium of KNa/NaL » k¢/k2¢[L]org and the above k¢/[L]org and KNa/NaL values, the k2¢ values were estimated to be 0.050min.−1 for L=B18C6 with NB, 4 for B15C5 with DCE, and 0.70 for B18C6 with DCE. Only the obtained k2¢ value for B18C6 with NB was close to that (0.056min.−1 at 298K13) for B18C6 with CH2Cl2. The corresponding pseudo-second-order-reaction rate-constants k¢/[L]org were 1.5´102mol−1 dm3 min.−1, 8.8, and 13, respectively. These facts simply show k¢/[L]org >k2¢, although their units differ from each other. On the other hand, we can derive the limitation of k1>k2 from Eq. (8a), since both the relations of KNa/NaL>1 and k¢>0 holds (see the above results). This accordance between the inequalities suggests that k1/k2 is proportional to k¢/k2¢[L]org.
For the rate of the ion transfer from the org to water phase
Assuming that the relation of -d[NaL+]/dt=k[NaL+]−kb[NaL+]org holds, we can easily estimate the apparent backward rate-constant kb from the relation26 of KD,NaL=k/kb at equilibrium. The estimated kb values were 1.5´10-5 min.-1 at L=15C5, 6.6´10-5 and 7.0´10-5 at B15C5, 1.5´10-5 at 18C6, and 1.0´10-6 at B18C6 for the NB systems and 0.01 at 15C5, 3´10-4 at B15C5, 0.0064 at 18C6, and 9.6´10-7 at B18C6 for the DCE ones. One can see that the relation k>kb holds in all the L and satisfies the assumption 2 (see the theoretical handling section), although the 15C5 system with DCE does not adequately satisfy the assumption 3 of KD>>1 (see the KD,Na15C5=2.5 in Table 1). These results suggest that the NaL+ transfers from the org phase to the water one are slow, compared with the transfer from water phase to the org one. It is interesting that the process of NaL+org→Lorg+Na+ is very fast (see the k2¢ values), compared with that of NaL+org→NaL+ (see the kb ones) at L=B15C5 and B18C6. This fact suggests that the former process is dominant for the release of the Na(I) species from the org phase II to the receiving phase I with TBA+Cl−, compared with the latter process.
The apparent k values were in the range of 10−3 to 10−2min.−1 at 298K for the liquid membrane transport of the Na(I) species with L, probably NaL+. Also, the kb values were estimated to be 10−6 to 10-2min.−1. For the Na(I) release from the NB phase II to the water phase I, it was suggested that the process of NaB18C6+NB→B18C6NB+Na+ is more speedy than that of NaB18C6+NB→NaB18C6+. The same is true of the B15C5 and B18C6 systems with DCE. Besides, the good correlations of the dep-versus-ln KD¢ plots indirectly confirmed the presence of the dep between the two bulk phases for the liquid-membrane transport systems, as well as extraction systems.
As shown in the materials and methods section, the study proposes a simple procedure which monitors emf with t. This gives the possibility that many unprofessional workers can do thus transport experiments, because the experiments do not need comparatively expensive instruments, such as AAS13–15,17 and HPLC.19 On the other hand, needless to say, the neutral species, such as NaLPic and NaPic, cannot be detected with this method. This limitation becomes a weak point of the method, as well as the traditional methods reported before. This study should be acceptable for readers as primary experiments. Additional experiments will be required for confirming these results more directly, if possible.
None.
The author declares that there is no conflict of interest.

©2018 Kudo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.