Journal of
eISSN: 2473-0831


Research Article Volume 7 Issue 5
Correspondence: Shipra Baluja, Department of Chemistry, Saurashtra University, Rajkot-360005 (India), Tel -9687692827
Received: July 30, 2017 | Published: September 14, 2018
Citation: Baluja S, Hirapara A, Lava D. Physicochemical properties of some pyrimidine derivatives in some organic solvents. J Anal Pharm Res. 2018;7(5):540-546. DOI: 10.15406/japlr.2018.07.00280
Some physicochemical parameters such as density, refractive index and conductance of some newly synthesized pyrimidine derivatives have been measured in N, N-dimethyl formamide, chloroform and methanol at 308.15K. It is observed that these studied parameters depend on the solvent and structure of compounds, which may be due to different type of interactions.
Keywords: density, refractive index, conductance, methanol, N, N-dimethyl formamide, chloroform
Heterocyclic compounds play an immense role in many biochemical processes1 and numerous heterocyclic compounds are biosynthesized by plants and animals, which are also associated to significant biological properties. Nitrogen containing heterocyclic compounds are known to play an essential role in many living systems. The nucleic acid bases are the derivatives of pyrimidine and purine,2 found in RNA and DNA in the form of uracil, thymine, cytosine, adenine and guanine. These nitrogen containing heterocycles are synthetically challenging models for a number of physiologically active natural products.3
Pyrimidines are always an attraction point for researchers due to their pharmacological usages. These compounds are known to possess wide spectrum of biological activities such as anti-tubercular, anti-HIV, anti-microbial, anti-analgesic, anti-inflammatory and anti-malarial, antidepressant, anticonvulsant, antioxidant, anticancer, antifungal, etc.4–16.
Thus, due to these biologically activity of pyrimidines, in the present paper, different pyrimidines compounds i.e., tetrahydropyrimidines and 2, 4-disubstituted pyrimidines have been synthesized. Some physicochemical properties such as density, refractive index and conductance of solutions of these synthesized compounds have been studied in different solvents at 308.15K.
Some new tetrahydropyrimidines and 2, 4-disubstituted pyrimidines compounds have been synthesized. The general structures and substitutions in different compounds are given in Figure 1.
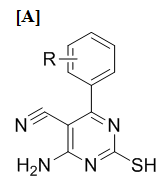
Where R is : SNS-1: 4-OH, 3-OCH3-C6H4; SNS-2: 4-OCH3-C6H4; SNS-3: 4-OH-C6H4; SNS-4: 4-Cl-C6H4; SNS-5: 3-Cl-C6H4; SNS-6: 4-F-C6H4; SNS-7: 3-NO2-C6H4; SNS-8: -C6H5; SNS-9: C4H3O; SNS-10: -CH=CH-C6H5;

Where R is: SDN-1: 4-Cl; SDN-2: 4-CH3; SDN-3: 4-F; SDN-4: 3-CF3; SDN-5: 3-Cl, 4-F;

Where R is: SDO-1: 4-Cl; SDO-2: 4-CH3; SDO-3: 4-F; SDO-4: 3-CF3; SDO-5: 3-Cl, 4-F.
Figure 1 General structure of synthesized different pyrimidine derivatives: [A] tetrahydropyrimidines (SNS series); [B] 2, 4-disubstituted pyrimidines (SDN series) and [C] 2, 4-disubstituted pyrimidines (SDO series)
Physicochemical studies
The solvents DMF, chloroform and methanol used for physicochemical studies were purified by fractionally distillation by the reported method.17 For each compound, a series of solutions of different concentrations were prepared in these solvents. The choice of different solvents for different compounds is due to solubility problem.
The density and refractive index of pure solvents and solutions were measured by using pycnometer and Abbe refractometer respectively at 308.15K. The desired temperature was maintained by circulating water through jacket around the prisms of refractometer from an electronically controlled thermostatic water bath (NOVA NV-8550 E). The uncertainty of temperature was ±0.1°C.
The conductance of each solution was measured by using Elico Conductivity Meter (Model No. CM 180) at 308.15K. The measured conductance was corrected by subtracting the conductance of pure solvent.
Density and refractive index
Table 1 shows the experimental values of densities and refractive index for all the studied solutions. Using experimental density of solution, density of each compound was calculated using the following relation:
(1)
Conc. M |
ρ12 g.cm-3 |
n |
ρ12 g.cm-3 |
N |
Tetrahydropyrimidines |
||||
|
DMF |
Chloroform |
||
SNS-1 |
||||
0.00 |
0.9338 |
1.4239 |
1.4713 |
1.4397 |
0.01 |
0.9456 |
1.4231 |
1.4715 |
1.4402 |
0.02 |
0.9465 |
1.4239 |
1.4718 |
1.4410 |
0.04 |
0.9483 |
1.4247 |
1.4721 |
1.4421 |
0.06 |
0.9500 |
1.4255 |
1.4726 |
1.4433 |
0.08 |
0.9516 |
1.4265 |
1.4731 |
1.4448 |
0.10 |
0.9535 |
1.4273 |
1.4737 |
1.4458 |
SNS-2 |
||||
0.01 |
0.9448 |
1.4223 |
1.4717 |
1.4399 |
0.02 |
0.9452 |
1.4229 |
1.4724 |
1.4404 |
0.04 |
0.9461 |
1.4238 |
1.4736 |
1.4414 |
0.06 |
0.9470 |
1.4248 |
1.4749 |
1.4423 |
0.08 |
0.9479 |
1.4259 |
1.4761 |
1.4433 |
0.10 |
0.9489 |
1.4268 |
1.4774 |
1.4443 |
SNS-3 |
||||
0.01 |
0.9486 |
1.4212 |
1.4714 |
1.4400 |
0.02 |
0.9489 |
1.4228 |
1.4718 |
1.4406 |
0.04 |
0.9495 |
1.4230 |
1.4727 |
1.4417 |
0.06 |
0.9501 |
1.4242 |
1.4736 |
1.4428 |
0.08 |
0.9507 |
1.4252 |
1.4745 |
1.4440 |
0.10 |
0.9513 |
1.4263 |
1.4754 |
1.4450 |
SNS-4 |
||||
0.01 |
0.9494 |
1.4219 |
1.4749 |
1.4415 |
0.02 |
0.9503 |
1.4223 |
1.4753 |
1.4416 |
0.04 |
0.9520 |
1.4231 |
1.4761 |
1.4420 |
0.06 |
0.9538 |
1.4239 |
1.4768 |
1.4423 |
0.08 |
0.9555 |
1.4250 |
1.4776 |
1.4425 |
0.10 |
0.9573 |
1.4255 |
1.4784 |
1.4428 |
SNS-5 |
||||
0.01 |
0.9494 |
1.4203 |
1.4720 |
1.4409 |
0.02 |
0.9503 |
1.4208 |
1.4723 |
1.4410 |
0.04 |
0.9520 |
1.4217 |
1.4730 |
1.4413 |
0.06 |
0.9538 |
1.4226 |
1.4736 |
1.4416 |
0.08 |
0.9556 |
1.4235 |
1.4743 |
1.4418 |
0.10 |
0.9573 |
1.4244 |
1.4750 |
1.4421 |
SNS-6 |
||||
0.01 |
0.9454 |
1.4211 |
1.4747 |
1.4412 |
0.02 |
0.9458 |
1.4215 |
1.4752 |
1.4414 |
0.04 |
0.9467 |
1.4222 |
1.4762 |
1.4418 |
0.06 |
0.9476 |
1.4230 |
1.4772 |
1.4425 |
0.08 |
0.9484 |
1.4237 |
1.4782 |
1.4426 |
0.10 |
0.9494 |
1.4244 |
1.4792 |
1.4430 |
SNS-7 |
||||
0.01 |
0.9529 |
1.4191 |
1.4800 |
1.4408 |
0.02 |
0.9530 |
1.4200 |
1.4801 |
1.4412 |
0.04 |
0.9531 |
1.4217 |
1.4803 |
1.4420 |
0.06 |
0.9535 |
1.4234 |
1.4805 |
1.4426 |
0.08 |
0.9537 |
1.4252 |
1.4807 |
1.4433 |
0.10 |
0.9540 |
1.4269 |
1.4809 |
1.4440 |
SNS-8 |
||||
0.01 |
0.9506 |
1.4225 |
1.4735 |
1.4408 |
0.02 |
0.9517 |
1.4228 |
1.4737 |
1.4411 |
0.04 |
0.9528 |
1.4234 |
1.4741 |
1.4416 |
0.06 |
0.9531 |
1.4239 |
1.4744 |
1.4421 |
0.08 |
0.9547 |
1.4245 |
1.4748 |
1.4426 |
0.10 |
0.9551 |
1.4251 |
1.4751 |
1.4431 |
SNS-9 |
||||
0.01 |
0.9424 |
1.4164 |
1.4809 |
1.4397 |
0.02 |
0.9429 |
1.4175 |
1.4813 |
1.4400 |
0.04 |
0.9440 |
1.4195 |
1.4820 |
1.4406 |
0.06 |
0.9451 |
1.4214 |
1.4827 |
1.4412 |
0.08 |
0.9462 |
1.4234 |
1.4834 |
1.4418 |
0.10 |
0.9473 |
1.4253 |
1.4741 |
1.4424 |
SNS-10 |
||||
0.01 |
0.9562 |
1.4233 |
1.4746 |
1.4396 |
0.02 |
0.9565 |
1.4238 |
1.4751 |
1.4398 |
0.04 |
0.9571 |
1.4244 |
1.4763 |
1.4399 |
0.06 |
0.9576 |
1.4251 |
1.4775 |
1.4401 |
0.08 |
0.9581 |
1.4257 |
1.4787 |
1.4402 |
0.10 |
0.9587 |
1.4262 |
1.4798 |
1.4404 |
2, 4-disubstituted pyrimidines |
||||
|
DMF |
Methanol |
||
SDN-1 |
||||
0.00 |
0.9338 |
1.4239 |
0.7770 |
1.3250 |
0.01 |
0.9432 |
1.4245 |
0.7794 |
1.3260 |
0.02 |
0.9446 |
1.4255 |
0.7812 |
1.3265 |
0.04 |
0.9468 |
1.4270 |
0.7839 |
1.3295 |
0.06 |
0.9484 |
1.4285 |
0.7879 |
1.3310 |
0.08 |
0.9497 |
1.4295 |
0.7892 |
1.3325 |
0.10 |
0.9516 |
1.4310 |
0.7909 |
1.3330 |
SDN-2 |
||||
0.01 |
0.9430 |
1.4240 |
0.7816 |
1.3265 |
0.02 |
0.9441 |
1.4245 |
0.7839 |
1.3280 |
0.04 |
0.9462 |
1.4260 |
0.7880 |
1.3300 |
0.06 |
0.9479 |
1.4270 |
0.7915 |
1.3315 |
0.08 |
0.9491 |
1.4285 |
0.7948 |
1.3330 |
0.10 |
0.9504 |
1.4300 |
0.7997 |
1.3345 |
SDN-3 |
||||
0.01 |
0.9428 |
1.4250 |
0.7804 |
1.3280 |
0.02 |
0.9438 |
1.4260 |
0.7819 |
1.3295 |
0.04 |
0.9455 |
1.4275 |
0.7837 |
1.3315 |
0.06 |
0.9474 |
1.4295 |
0.7869 |
1.3330 |
0.08 |
0.9489 |
1.4310 |
0.7883 |
1.3345 |
0.10 |
0.9501 |
1.4320 |
0.7893 |
1.3360 |
SDN-4 |
||||
0.01 |
0.9434 |
1.4250 |
0.7830 |
1.3265 |
0.02 |
0.9447 |
1.4265 |
0.7851 |
1.3285 |
0.04 |
0.9474 |
1.4285 |
0.7883 |
1.3310 |
0.06 |
0.9506 |
1.4300 |
0.7907 |
1.3325 |
0.08 |
0.9520 |
1.4315 |
0.7932 |
1.3345 |
0.10 |
0.9541 |
1.4325 |
0.7969 |
1.3380 |
SDN-5 |
||||
0.01 |
0.9432 |
1.4255 |
0.7798 |
1.3265 |
0.02 |
0.9445 |
1.4270 |
0.7821 |
1.3280 |
0.04 |
0.9470 |
1.4285 |
0.7860 |
1.3295 |
0.06 |
0.9492 |
1.4300 |
0.7889 |
1.3330 |
0.08 |
0.9512 |
1.4320 |
0.7921 |
1.3360 |
0.10 |
0.9525 |
1.4315 |
0.7958 |
1.3405 |
|
DMF |
Methanol |
||
SDO-1 |
||||
0.01 |
0.9425 |
1.4245 |
0.7791 |
1.3265 |
0.02 |
0.9440 |
1.4260 |
0.7802 |
1.3270 |
0.04 |
0.9467 |
1.4275 |
0.7824 |
1.3275 |
0.06 |
0.9490 |
1.4280 |
0.7847 |
1.3280 |
0.08 |
0.9517 |
1.4300 |
0.7868 |
1.3290 |
0.10 |
0.9529 |
1.4320 |
0.7894 |
1.3305 |
SDO-2 |
||||
0.01 |
0.9422 |
1.4240 |
0.7793 |
1.3260 |
0.02 |
0.9434 |
1.4245 |
0.7809 |
1.3270 |
0.04 |
0.9449 |
1.4255 |
0.7831 |
1.3275 |
0.06 |
0.9488 |
1.4265 |
0.7857 |
1.3285 |
0.08 |
0.9502 |
1.4280 |
0.7872 |
1.3294 |
0.10 |
0.9513 |
1.4295 |
0.7912 |
1.3315 |
SDO-3 |
||||
0.01 |
0.9428 |
1.4240 |
0.7795 |
1.3260 |
0.02 |
0.9447 |
1.4245 |
0.7809 |
1.3275 |
0.04 |
0.9461 |
1.4255 |
0.7829 |
1.3290 |
0.06 |
0.9496 |
1.4260 |
0.7855 |
1.3305 |
0.08 |
0.9520 |
1.4270 |
0.7876 |
1.3315 |
0.10 |
0.9535 |
1.4281 |
0.7914 |
1.3335 |
SDO-4 |
||||
0.01 |
0.9429 |
1.4240 |
0.7801 |
1.3265 |
0.02 |
0.9452 |
1.4245 |
0.7824 |
1.3260 |
0.04 |
0.9471 |
1.4255 |
0.7864 |
1.3270 |
0.06 |
0.9489 |
1.4270 |
0.7898 |
1.3280 |
0.08 |
0.9507 |
1.4285 |
0.7923 |
1.3295 |
0.10 |
0.9530 |
1.4295 |
0.7950 |
1.3330 |
SDO-5 |
||||
0.01 |
0.9431 |
1.4245 |
0.7797 |
1.3260 |
0.02 |
0.9449 |
1.4265 |
0.7814 |
1.3275 |
0.04 |
0.9468 |
1.4290 |
0.7844 |
1.3285 |
0.06 |
0.9495 |
1.4315 |
0.7869 |
1.3295 |
0.08 |
0.9520 |
1.4325 |
0.7885 |
1.3305 |
0.10 |
0.9540 |
1.4340 |
0.7923 |
1.3315 |
Table 1 The density (ρ12) and refractive index (n) of synthesized compounds at 308.15K
where ρ12 is the density of solution and ρ1 and ρ2 are the densities of solvent and solute respectively. g1 and g2are the weight fractions of solvent and solute.
The evaluated densities of all the compounds are listed in Table 2 along with the theoretical densities, which were calculated using the following equation:18
(2)
Compounds |
Experimental density g.cm-3 |
Theoretical density g.cm-3 |
|
|
DMF |
Chloroform |
|
SNS-1 |
1.5175 |
1.6234 |
1.2960 |
SNS-2 |
1.2270 |
1.9380 |
1.2624 |
SNS-3 |
1.2870 |
1.7825 |
1.3163 |
SNS-4 |
1.7953 |
1.8692 |
1.3565 |
SNS-5 |
1.7699 |
1.6978 |
1.3565 |
SNS-6 |
1.2610 |
2.0121 |
1.3351 |
SNS-7 |
1.2438 |
1.8519 |
1.3664 |
SNS-8 |
1.2315 |
1.6892 |
1.2787 |
SNS-9 |
1.2642 |
2.4213 |
1.5300 |
SNS-10 |
1.5974 |
2.0661 |
1.3877 |
|
DMF |
Methanol |
|
SDN-1 |
1.2804 |
1.2484 |
1.3612 |
SDN-2 |
1.2531 |
1.9417 |
1.2766 |
SDN-3 |
1.3405 |
1.1628 |
1.3485 |
SDN-4 |
1.3605 |
1.3680 |
1.6028 |
SDN-5 |
1.3021 |
1.4599 |
1.4420 |
SDO-1 |
1.4368 |
1.1919 |
1.1947 |
SDO-2 |
1.3870 |
1.3123 |
1.1177 |
SDO-3 |
1.4306 |
1.7007 |
1.1787 |
SDO-4 |
1.3228 |
1.2063 |
1.3514 |
SDO-5 |
1.4225 |
1.2887 |
1.2192 |
Table 2 Experimental and theoretical densities of compounds at 308.15K
where ρ is the density of the compound, K is packing fraction (0.599), M is the molecular weight of the compound, NA is the Avogadro’s number and ΔVi is the volume increment of the atoms and atomic groups present in the compound.
Comparison of densities values showed that theoretical density values are different from those evaluated from experimental data. Further, for the same compound, density in the two solvents is different. This suggests that solvent plays an important role. In solutions, compounds interact differently depending upon their substitution, structure and nature of solvent. These molecular interactions affect volume, which causes change in density.
Further, the molar refraction of a pure liquid (MRD)1 were calculated by the following equation:
(3)
where n, M and ρ are refractive index, molecular weight and density pure liquid respectively.
For solutions, the following equation was used to determining molar refraction:
(4)
where n12 and ρ12 are refractive index and density of solution respectively. X1 and X2 are the mole fractions and M1 and M2 are the molecular weight of the solvent and solute respectively.
From the values of the molar refraction of solution and pure solvent, molar refraction of solid compounds were determined by following equation:
(5)
From the density and molar refraction data, the refractive indexes of all the compounds were calculated from eq. (5). The molar refraction (MRD)2 and refractive index of all the compounds are reported in Table 3 for 0.1M solution.
Compounds |
(MRD2) |
n |
(MRD2) |
N |
Solvents |
||||
|
DMF |
Chloroform |
||
SNS-1 |
121.62 |
1.7680 |
76.53 |
1.4703 |
SNS-2 |
127.01 |
1.6448 |
59.26 |
1.4469 |
SNS-3 |
114.30 |
1.5263 |
64.01 |
1.3921 |
SNS-4 |
99.00 |
1.7553 |
50.46 |
1.3539 |
SNS-5 |
94.42 |
1.7361 |
53.75 |
1.3576 |
SNS-6 |
109.83 |
1.5803 |
47.02 |
1.3722 |
SNS-7 |
119.41 |
1.5820 |
54.05 |
1.3677 |
SNS-8 |
104.12 |
1.5317 |
51.63 |
1.3419 |
SNS-9 |
112.69 |
1.6026 |
30.06 |
1.2799 |
SNS-10 |
96.77 |
1.6999 |
33.79 |
1.2784 |
|
DMF |
Methanol |
||
SDN-1 |
116.23 |
1.7175 |
107.01 |
1.6251 |
SDN-2 |
108.67 |
1.6872 |
87.16 |
1.9230 |
SDN-3 |
121.28 |
1.8583 |
124.15 |
1.7308 |
SDN-4 |
126.43 |
1.7764 |
128.42 |
1.7989 |
SDN-5 |
121.29 |
1.7296 |
141.56 |
2.0683 |
SDO-1 |
109.87 |
1.8569 |
88.87 |
1.5211 |
SDO-2 |
95.38 |
1.7731 |
84.56 |
1.5932 |
SDO-3 |
82.26 |
1.6053 |
86.98 |
1.8493 |
SDO-4 |
122.39 |
1.9435 |
106.60 |
1.5894 |
SDO-5 |
105.28 |
1.6510 |
91.59 |
1.5586 |
Table 3 Molar refraction (MRD)2 and refractive index (n) of 0.1M solutions of compounds at 308.15K
It is evident from Table 3 that both (MRD)2 and refractive index of compounds are different in each solvent. This again proves that in different solvents, intermolecular interactions are different, which affect these parameters. In some solvents, there may be interaction between solute and solvent molecules where as in others breakage of bonds may take place. As refractive index and molar refraction depends upon not only atomic refraction but also upon single, double or triple bonds, the type of interactions taking place in solution affects these parameters. Further, bond polarity also causes change in molar refraction. Thus, type of solvent affects the refractive index and molar refraction of a solute.
Conductance
The measured conductance (k) of each solution after correction are given in Table 4. It is observed that for all the studied compounds, conductance increases with concentration in all the solvents. The conductance measurement of two tetrahydropyrimidine compounds SNS-1 and SNS-3 cannot be done as these compounds had very less solubility in chloroform. For both tetrahydropyrimidines 2, 4-disubstituted pyrimidine compounds, conductance is lower in DMF than that in chloroform and methanol respectively.
Conc. |
k.104mho |
|||||||||
Tetrahydropyrimidines |
||||||||||
DMF |
||||||||||
|
SNS -1 |
SNS -2 |
SNS -3 |
SNS -4 |
SNS -5 |
SNS -6 |
SNS -7 |
SNS -8 |
SNS -9 |
SNS -10 |
0.000 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.001 |
0.0187 |
0.0174 |
0.0017 |
0.0089 |
0.0050 |
0.0017 |
0.0013 |
0.0122 |
0.0030 |
0.0209 |
0.002 |
0.0370 |
0.0346 |
0.0034 |
0.0169 |
0.0094 |
0.0028 |
0.0021 |
0.0238 |
0.0059 |
0.0414 |
0.004 |
0.0728 |
0.0684 |
0.0064 |
0.0326 |
0.0170 |
0.0057 |
0.0040 |
0.0454 |
0.0115 |
0.0804 |
0.006 |
0.1062 |
0.0988 |
0.0094 |
0.0463 |
0.0243 |
0.0077 |
0.0053 |
0.0660 |
0.0165 |
0.1140 |
0.008 |
0.1336 |
0.1284 |
0.0111 |
0.0580 |
0.0295 |
0.0088 |
0.0051 |
0.0843 |
0.0184 |
0.1424 |
0.010 |
0.1420 |
0.1526 |
0.0075 |
0.0675 |
0.0306 |
0.0100 |
0.0043 |
0.1000 |
0.0190 |
0.1550 |
0.020 |
0.1979 |
0.2453 |
0.0279 |
0.1121 |
0.0494 |
0.0291 |
0.0106 |
0.1421 |
0.0352 |
0.2467 |
0.040 |
0.2246 |
0.3684 |
0.0470 |
0.2020 |
0.0730 |
0.0684 |
0.0198 |
0.1876 |
0.0620 |
0.3206 |
0.060 |
0.2495 |
0.4080 |
0.1009 |
0.2735 |
0.0726 |
0.0996 |
0.0178 |
0.2331 |
0.0720 |
0.3947 |
0.080 |
0.2738 |
0.3449 |
0.1258 |
0.3459 |
0.0746 |
0.1299 |
0.0176 |
0.2779 |
0.0756 |
0.4689 |
0.100 |
0.2992 |
0.2707 |
0.1595 |
0.4185 |
0.0709 |
0.1586 |
0.0107 |
0.3241 |
0.0783 |
0.5431 |
Chloroform |
||||||||||
0.000 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.084 |
0.001 |
- |
0.0890 |
- |
0.0890 |
0.0850 |
0.1000 |
0.1050 |
0.1038 |
0.1246 |
0.1187 |
0.002 |
- |
0.1740 |
- |
0.1558 |
0.1335 |
0.1880 |
0.1860 |
0.2003 |
0.2373 |
0.2225 |
0.004 |
- |
0.3400 |
- |
0.2967 |
0.2373 |
0.3560 |
0.3227 |
0.3560 |
0.4450 |
0.4050 |
0.006 |
- |
0.4980 |
- |
0.3900 |
0.3120 |
0.4680 |
0.4160 |
0.4800 |
0.6000 |
0.5680 |
0.008 |
- |
0.6240 |
- |
0.5200 |
0.3740 |
0.5280 |
0.5020 |
0.5512 |
0.7390 |
0.6760 |
0.010 |
- |
0.6400 |
- |
0.4450 |
0.4020 |
0.5500 |
0.5120 |
0.5680 |
0.8460 |
0.7400 |
0.020 |
- |
0.8900 |
- |
0.7000 |
0.6230 |
0.8900 |
0.7120 |
1.0680 |
1.2460 |
1.1570 |
0.040 |
- |
0.9790 |
- |
0.8010 |
0.6230 |
0.8900 |
0.8010 |
1.2460 |
1.7350 |
1.6460 |
0.060 |
- |
0.9790 |
- |
0.8900 |
0.7120 |
0.9790 |
0.8900 |
1.3350 |
1.9350 |
1.8000 |
0.080 |
- |
1.0680 |
- |
0.8900 |
0.7120 |
0.9790 |
0.9790 |
1.4240 |
2.0640 |
1.6800 |
0.100 |
- |
1.0680 |
- |
0.9790 |
0.8010 |
0.9790 |
1.1570 |
1.6020 |
1.9900 |
1.5130 |
2,4-Disubstituted pyrimidines |
||||||||||
DMF |
||||||||||
|
SDN-1 |
SDN -2 |
SDN -3 |
SDN -4 |
SDN -5 |
SDO -1 |
SDO-2 |
SDO-3 |
SDO-4 |
SDO-5 |
0.000 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.2800 |
0.001 |
0.0735 |
0.2037 |
0.1200 |
0.1851 |
0.0828 |
0.0642 |
0.1107 |
0.2967 |
0.1851 |
0.2595 |
0.002 |
0.2130 |
0.3525 |
0.2874 |
0.3060 |
0.2316 |
0.2781 |
0.3339 |
0.5757 |
0.4827 |
0.5850 |
0.004 |
0.4362 |
0.6780 |
0.5943 |
0.5385 |
0.4362 |
0.6687 |
0.6780 |
1.0221 |
1.0128 |
1.1988 |
0.006 |
0.6036 |
0.9105 |
0.8361 |
0.7803 |
0.5943 |
1.0221 |
0.9849 |
1.4313 |
1.3941 |
1.6452 |
0.008 |
0.7802 |
1.1058 |
1.0593 |
0.9662 |
0.7524 |
1.3569 |
1.2360 |
1.8218 |
1.8405 |
2.0544 |
0.010 |
0.8547 |
1.3197 |
1.4127 |
1.1523 |
0.9291 |
1.7010 |
1.4871 |
2.2497 |
2.2404 |
2.3241 |
0.020 |
1.3940 |
2.1938 |
2.0916 |
1.5986 |
1.4778 |
2.8262 |
2.3612 |
3.6726 |
4.3700 |
3.9702 |
0.040 |
2.2684 |
3.0868 |
3.3284 |
2.4076 |
2.4544 |
3.9700 |
4.1656 |
6.4440 |
6.8532 |
5.4116 |
0.060 |
2.6496 |
3.6540 |
3.9330 |
3.1890 |
3.2262 |
5.3928 |
5.2440 |
8.1828 |
7.6530 |
6.3138 |
0.080 |
3.0216 |
4.2960 |
4.1656 |
3.7008 |
4.0072 |
6.3512 |
5.8952 |
9.2896 |
8.0992 |
7.2064 |
0.100 |
3.2630 |
4.4540 |
4.2310 |
4.1840 |
4.2030 |
6.8900 |
6.4250 |
9.5220 |
8.6020 |
8.3500 |
Methanol |
||||||||||
0.000 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.0400 |
0.001 |
0.0558 |
0.4929 |
0.0837 |
0.0651 |
0.0372 |
0.0558 |
0.0465 |
0.8928 |
0.9951 |
0.7254 |
0.002 |
0.2511 |
0.9021 |
0.4278 |
0.6045 |
0.5115 |
0.2465 |
0.5673 |
1.9158 |
1.9902 |
1.7112 |
0.004 |
0.7719 |
1.7949 |
1.2369 |
1.5066 |
1.4415 |
0.8928 |
1.5252 |
3.8967 |
3.7851 |
3.5247 |
0.006 |
1.2927 |
2.5854 |
1.9065 |
2.4924 |
2.2599 |
1.4880 |
2.5017 |
5.7195 |
5.7102 |
5.0406 |
0.008 |
1.8414 |
3.3759 |
2.7528 |
3.3108 |
3.1713 |
2.0181 |
3.3945 |
7.6818 |
7.3842 |
6.0450 |
0.010 |
2.2320 |
3.9339 |
3.3108 |
4.1943 |
4.3617 |
2.5017 |
4.6128 |
9.0954 |
8.7234 |
7.6167 |
0.020 |
4.0827 |
7.5516 |
6.2589 |
8.0538 |
7.8492 |
4.0827 |
8.5002 |
15.9774 |
15.1404 |
12.6294 |
0.040 |
6.5100 |
13.0014 |
11.2344 |
14.0244 |
13.0014 |
10.0347 |
13.9314 |
25.5564 |
24.9984 |
21.0924 |
0.060 |
9.6813 |
19.1394 |
15.9774 |
19.2324 |
17.7444 |
14.1174 |
19.3254 |
34.2984 |
33.8334 |
27.8814 |
0.080 |
12.0807 |
23.0454 |
22.5804 |
23.7894 |
21.8364 |
18.0234 |
23.8824 |
42.6684 |
40.5294 |
33.8334 |
0.100 |
15.3264 |
27.9744 |
25.8354 |
25.9284 |
25.7424 |
24.4404 |
28.9044 |
49.2714 |
45.6444 |
38.3904 |
Table 4 The conductance (k) of synthesized compounds in different solvents at 308.15K
From corrected conductance, specific conductance (κ) and equivalent conductance (λc) are calculated using the following equations:
(6)
(7)
where θ is the cell constant (0.96cm-1) and c is the concentration (g.equi./lit.) of solution.
The equivalent conductance (λc) is plotted against √C for all compounds as shown in Figure 2-4. For tetrahydropyrimidine compounds, in DMF, equivalent conductance increases with concentration in both the solvents. At higher concentrations, the variation of equivalent conductance for different compounds is very less. Further, in DMF, equivalent conductance for tetrahydropyrimidine compounds are much lower than those in chloroform. It is obvious from Figure 2 that in DMF, most of tetrahydropyrimidine compounds behave as weak electrolytes whereas in chloroform, these compounds exhibited electrolytic behavior. For 2, 4-disubstituted pyrimidine compounds (both SDN and SDO compounds) also, equivalent conductance is less in DMF solutions than those in methanol solutions. Figure 3 shows that in DMF, SDN-2 and SDN-4 showed electrolytic behavior whereas for SDN-1, SDN-3 and SDN-5 compounds, equivalent conductance decreases at lower concentration. In methanol also, except SDN-2, for other four compounds also equivalent conductance decreases at lower concentration. Similar behavior was also observed for SDO compounds in both DMF and methanol solutions except SDO-3 in DMF (Figure 4). This typical behavior may be due to interactions within the molecule thereby causing constriction within the molecule or due to association between solute with solvent molecules. Similar behavior was observed by Singh et al.19,20
It is observed that physicochemical parameters of compounds in solution depends not only on the structure and substitution of the compound but also on the nature of solvent in which it is dissolved. The molecular interactions occurring in the solution affect volume which in turn causes a small change in density and refractive index. Depending upon the nature of solvent, the conductance i.e., electrolytic behavior of compounds also changes.
None.
The author declares that there is no conflict of interest.

©2018 Baluja, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.