The material and methods of this study is subdivided into two sections (2 and 3) respectively. Embedded in section 2, are the schematic representation of system model followed by derivation of model optimal control problem. We also show here that the state variables are non–negative and as well, ascertain the stability behavior of the system. Section 3, is devoted to the formulation of optimal control strategy and the optimality system of the model, which explores classical numerical methods known as Pontryagin maximum principle.
Schematic representation and model formulation
In structuring the progression of this model, we condone the fact that there are two virions (viral load and pathogen), which attack the immune system composing of two target cells (T–lymphocytes and macrophages). The scheme adopts the introduction of two chemotherapy cocktail (RTI and PIs) and taken into cognizance, natural interplay of adaptive immune effectors response. Thus, the model is constituted by 7 – subgroups, schematically represented as in Figure 1 below:
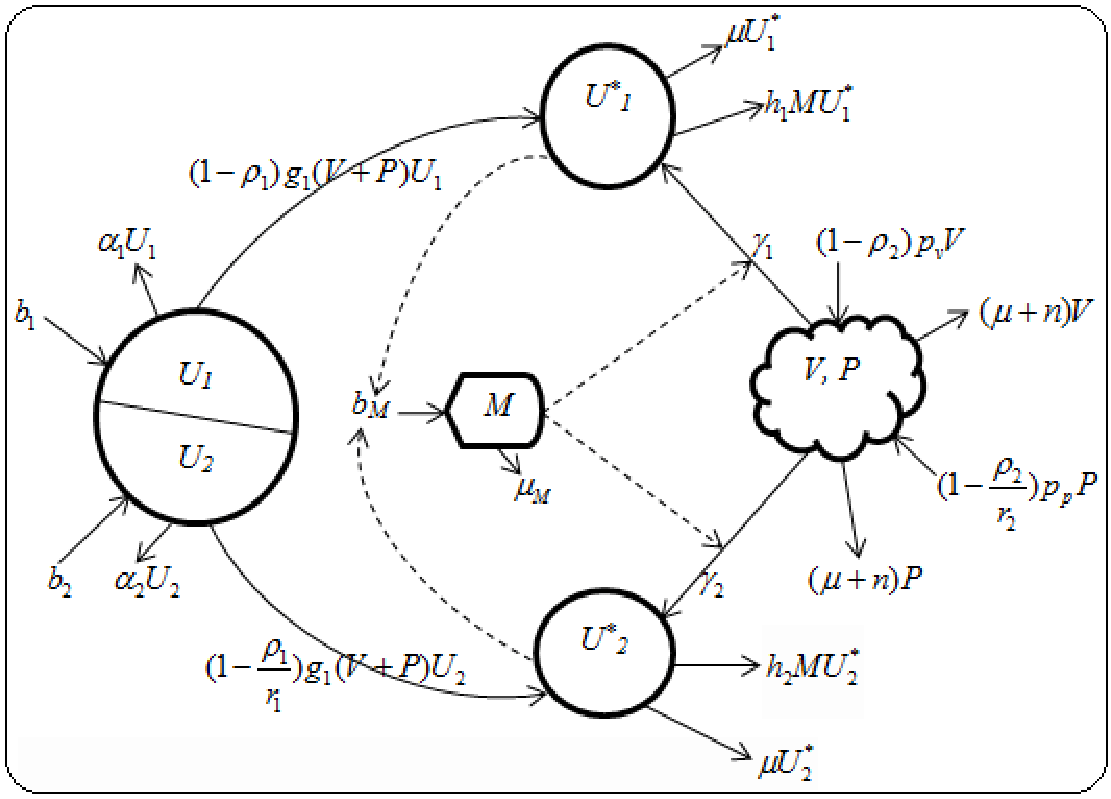
Figure 1 Schematic representation of dual HIV-pathogen infection with PMC treatment.
Obviously, from Figure 1, if these subgroups represent the population variables, with unit volume
, then we define as follows:
i=1, 2 as uninfected T–lymphocytes and macrophages cells,
, i=1, 2 as infected T–lymphocytes and macrophages cells, V – viral load, P – pathogen and M – immune effectors. Therefore, using classical ODEs, the simulative interactions of the above biological structure, is the formulation of a 7–Dimensional dynamic optimal control problem having its physiological derivation as follows:
(1)
(2)
(3)
(4)
(5)
(6)
(7)
with initial values
and
for all
and which satisfies the biological variables and parameter values as defined in Tables 1 & 2 below. The present model is in tune with the investigation by.20 where the author accounted for only single drug–RTI on single infection – HIV; and the study.1 accounted for chemotherapy “cocktail” (RTI and PIs) used for the study of dynamics of single infection–HIV.
|
Variables
|
Dependent Variables
|
|
Definition
|
Initial Values
|
Units
|
| U1 |
Uninfected T-lymph cells population
|
0.4
|
cells/mm3
|
| U2 |
Uninfected macrophages cells population
|
0.2
|
cells/mm3
|
| U*1 |
Infected T-lymph cells population
|
0.1
|
-
|
| U*2 |
Infected macrophages cells population
|
0.1
|
-
|
| V |
Infectious viral load population
|
0.2
|
mm3
|
| P |
Infectious pathogen population
|
0.1
|
mm3
|
| M |
Immune effectors response
|
10
|
mm3day-1
|
Table 1 Values used for state variables of model (2.1)-(2.7)
Laconically, equations (1)–(7) present the model of the system and the epidemiological terms are carefully defined thus: in equation (1),
– the source term of uninfected CD4+ T–lymphocytes and
– the death rate of uninfected T–lymphocytes cells. The term
is the interplay of viruses
with infectivity rate
, and
– drug efficacy, which model the RTI that block new infections, in
and
of the population. From equation (2),
– source term of uninfected macrophages cells,
– the death rate of uninfected macrophages cells. Here, the term
– represents the balance of treatment of viruses with infectivity rate
, on
and
– as treatment efficacy reduction in population
. The implication is that
and the ratio
constitutes the difference between the two cell populations. For these two cells, we assume that
, such that
and
denotes minimal and maximal drug efficacy respectively with
.
In equations (3) and (4), the first terms
and
are the results of the interactions of free CD4+ T–lymphocytes and macrophages with free virions
infectivity and the activation of proportionate (RTI) drug on infected
and
. The terms on infected
and
are the death rates of infected
and
. At the onset of infection, the third terms
, i=1, 2, represents the cytotoxic T–lymphatic (CTL) also known as CD8+ T cells, which exhibit the tendency of detecting and killing infected cells as much as possible. Therefore, in equations (3) and (4), terms
, i=1, 2, defines death of infected cells at the rate
, which depends on the density of immune effectors M.
(10)
From equation (5), the first term
defines the control function with
, representing the efficacy of protease inhibitors and having viral load multiplicative capacity
. The second term
defines reduction of infected cells due to clearance rate and the natural death rate of viral load. Thus, the productivity
is reduced to
, where
. The cumulative effect of viral load on both targeted cells in
and
is represented by the term
, which reveal
, i=1, 2 as probability of multiple virions infections on target cells
and
. In a chronological manner, equation (6) can be interpreted as in equation (5) but with respect to pathogenic infection and having differential effect of
as treatment reduction efficacy on
and
.
Remarkably, the clearance rate of infected cells.1 is conspicuously visible through the immune effector cells (CTL), designated by M, of equation (7). The dynamics of
is adopted from the study of.21 The term
– denotes the replication (or proliferation) of more effector cells from the co–existence of infected cell and immune effector cells. The second term
– defines the maximum birth rate of immune effectors in the presence of infected cells
,
and
; and having saturation birth constant
. The third term
is attributed to immune effector maximum death rate, where
is the effector death saturation constant. Lastly, the term
is the immune effectors natural death rate. Thus, the aforementioned descriptions are relevant in the administration of chemotherapy cocktail and PMC scenarios. Furthermore, the inclusion of immune effectors is a direct consequence of the sensitive role in the practical sense of PMC, which we will illustrate in the course of our numerical simulations.
From model (1)–(7) we observe that the function
,
, determines the percentage drug efficacy and the maximal use of chemotherapies. Thus,
is a measurable function having limit
on the domain
. This time interval accounts for the design of periodic treatment and as well, satisfies the objectives of this study as clearly mentioned in the introductory section. Furthermore, to ensure that our objective functional satisfies the control system, we take
such that
months of drugs allowable validity period.2,13,18
Therefore, the validity of the system equations (1)–(7) comes to bear, if we can genuinely generate compactible numerical values for the variables and parameters of the model. Attaining this, we adopt to the closest parameters values from those existing and valid studies as contain in our literature.1,14,17 Thus, the simulative parameter values that satisfy the biological variables and parameters of our model are explicitly defined as in Tables 1 & 2 below:
|
Parameter
|
Parameter and Constants
|
|
Definition
|
Initial Values
|
Units
|
| b1 |
Source of new T-lymph cells rate
|
100
|
cell/mm3.day
|
| b2 |
Source of new macrophages cells rate
|
20
|
cell/mm3.day
|
| α1 |
Death rate of uninfected T-lymph cells
|
0.02
|
day-1
|
| α2 |
Death rate of uninfected macrophages cells
|
0.02
|
day-1
|
| g1 |
Infected rate of T-lymph cells
|
0.004
|
mm3vir.-1d-1
|
| g2 |
Infected rate of macrophages cells
|
0.001
|
mm3vir.-1d-1
|
| ρ1 |
Treatment rate of RTI on U1,U*1,U2 and U*2
|
|
-
|
| ρ2 |
Treatment rate of PIs on V and P
|
|
-
|
| ri-1,2 |
Treatment efficacy reduction in U2 and U*2
|
0.14
|
-
|
| Pv |
Viral load multiplicity capacity
|
0.20
|
virions/cells
|
| Pp |
Macrophages multiplicity rate
|
0.04
|
virions/cells
|
| μ |
Infected virions death rate
|
0.07
|
day-1
|
| n |
Virions natural death rate
|
0.02
|
day-1
|
| γ1 |
Probability of multiple virions infection of T-lymph cells
|
1
|
virions/cells
|
| γ2 |
Probability of multiple virions infection of macrophages cells
|
1
|
virions/cells
|
| h1 |
Rate of death of infected T-lymph cells induced by immune effectors
|
0.004
|
mm3cell-1.d-1
|
| h2 |
Rate of death of infected macrophages cells induced by immune effectors
|
0.004
|
mm3cell-1.d-1
|
| bM |
Immune effectors replication rate
|
0.06
|
cell/mm3.day
|
| wM |
Immune effectors maximum birth rate
|
0.03
|
day-1
|
| Hw |
Immune effectors saturation birth rate
|
10
|
cells/mm
|
| qM |
Immune effectors maximum death rate
|
0.02
|
day-1
|
| Hq |
Immune effectors saturation death rate
|
30
|
cells/mm
|
| μM |
Immune effectors natural death rate
|
0.01
|
day-1
|
| C1 |
Half saturation constants for U*1,U*2
|
100
|
mm3
|
| C2 |
Half saturation constants for V,P
|
10
|
mm3
|
| C3 |
Half saturation constants for M
|
100
|
mm3
|
Table 2 Summary of model parameter values for (2.1)-(2.7)
Note: The table above is a reflection of models. 1,14,15,17 but clinically modified to accommodate the present novel model and compactible with RK4 software application utilized in this investigation.
Next, to satisfactorily establish the optimality and methodological application of our chemotherapy cocktail, it become necessary to first show that model state variables are non–negative and as well, discuss the disease stability analysis.
Positivity and compatibility of model variables
It is obvious that since maximal cost of chemotherapy cocktail is given by
, then possible drug severities is accounted for by the following proposition.
Proposition 2.1: If drug severities (or drug hazardous side–effects) emerges in the course of treatment, then the introduction of optimal weight factors
is justified. Also, if
represents minimal and maximal drugs efficacies, then the inequality
, i =1, 2 holds. Therefore, positivity for which model variables are compactible is studied using the following theorem.
Theorem 2.1: If equations (1)–(7) represents the model equations, such that for
, then the solution of the system
are non–negative and compactible for all
.22–24
Proof: We show that the state variables are life varying integers. Thus, invoking equations (1)–(7) step–wisely, we differentiating each of each them to justify their non–negativity.
From equation (1), we have
. Differentiating with respect to
, we obtain
. Since
, then
and is positive. The integrating factor IF is given by
. Multiplying the equation by the integrating factor, we have
. Rewriting the left hand side of the equation, we obtain
.
Integrating both sides, leads to
. Dividing by the integrating factor, we have
. Applying the initial condition i.e.
,
, then
,
. Therefore,
is non–negative and compactible. Next, taking equation (2), we set
Differentiating, we have,
and taking the integral gives
. Then applying the integrating factor
, we have
,
. Applying initial condition at
,
. Therefore, for
,
. Similarly, from equation (3), we have,
.
Differentiating, we see that
and taking the integral gives
. Applying the integrating factor
, we have
,
. Hence,
,
.
From equation (4), we have,
. Differentiating with respect to
, we obtain,
and taking the integral gives
. Applying the integrating factor
, we have
,
. When
, . Furthermore, taking equation (5), we set
Differentiating with respect to
, we obtain,
.
Taking the integral gives the expression
. Now, applying the integrating factor
, we have
,
. Therefore, when
,
.
Taking equation (6), we have
. Differentiating with respect to P, we obtain,
Taking the integral gives the expression
. Now, applying the integrating factor
, we have
,
. Therefore, when
,
.
Finally, from equation (7), we have,
.
Differentiating with respect to M, we obtain,
Taking the integral gives the expression
. Now, applying the integrating factor
, we have
,
. Therefore, when
, . Hence, all the model variables are non–negative and this completes the proof. We complete this section by highlighting the properties for which the PMC model is locally asymptotically stable.
Model stability analysis
Though the system stability analysis seems somewhat complex due to the nature of nonlinear systems involved, we’ll discuss the existing stability properties of the model in a simpler manner. Suppose,
defines the vectorial capacity of the model, then the system (1)–(7) satisfies the equation
(8)
Where
is the right–side of the ODE system and , the vector parameters as in Table 2. Then, we implore the Runge–Kutter of order 4, to solve the equation
for the steady states
. Next, we calculate the Jacobian matrix of the partial derivatives of the right side of the differential equations with respect to the state variables, i.e.
(9).
From equation (1)–(7), if we let
for the simple reason that we are poised to establish the system stability behavior when off treatment is applied, then the Jacobian matrix is derive as:
(10)
Where
and
.
So equation (10) exhibits non–singularity behavior since the diagonal of the Jacobian matrix is non–zero.
Then if we substitute the resulting computation of
steady state for v, in equation (10), we obtain the ODE system dynamics that is linearized about the equilibrium
. So we see from here that linearized ODE theory ascertain the fact that if the eigenvalues of the matrix have all negative real parts, then the equilibrium
is locally asymptotically stable. Therefore, given specific parameter values as in Tables 1 & 2, the model (1)–(7) exhibits three physical steady states and several non–physical steady states (omitted here for brevity). Thus, it is of interest to note that detail analysis of these stability behaviors is left to readers with related analysis as in model.1,8,25 This thought is in line with the focus of the present study – optimization control of PMC treatment and the maximization of CD4+ T cells and macrophages.
Unlike model.1 which considered single infection (HIV) in two immune systems with dual chemotherapy cocktail under STI program and which the method of analysis explored stability and linearization technique, the present model consider dual infectious variables under PMC program and focuses on utilizing classical numerical method known as Pontryagin’s maximum principle. This method allows the verification of existence of model as a function and the uniqueness of the system solution. To accomplish this task, we establish the model optimality control strategy from the derived optimal control problem.
Optimal control strategy and optimality system
Having shown that the model state variables are non–negative with known stability behavioral pattern, we then establish in this section, the optimal control strategy for continuous chemotherapy cocktail and the consequences following the introduction of optimal weight factors. This will lead to the derivation of existence of the model, establish the model optimality control system and lastly, prove the uniqueness of the solution of the system.
Optimal control strategy for continuous chemotherapy cocktail
We recall that optimal control strategy is the derivation of mathematical model (as in this case of model (1)–(7)) with which we define the objective functional as a function of maximization of the control variables. Indeed the objective functional of any optimal control problem is an integral equation, which model the trade–off between virions and pathogen concentration, organ health and use of therapeutics.26,27 Therefore, for a HIV–pathogen dynamics having optimal control problem as in equations (1)–(7), the objective functional that maximizes the control system is derive as:
(12)
where
are the control variables for RTIs and PIs respectively. The quantities
and δ are the optimal weight factors on the virions, control treatment inputs and immune effectors respectively. These control constants maximizes the system benefits quantified along the level of CD4+ T cells concentration as represented by the first and second terms of equation (12). The third and fourth terms of the equations accounts for the minimization of systemic cost of drugs treatment cocktail. So that, if
represents maxima drug usage, then periodic zero alternate application of multiple drug cocktail is visible. Therefore, the maximal cost of drug usage is given by
.28,29
The introduction of optimal weight factors
is a consequence of the fact that cost benefits are nonlinear. Thus, these serve as simple nonlinear controls on the system variables. And we can satisfactorily say that the objective functional (12) completely meets the aims of the present investigation, which is primed by maximization of immune systems and immune effector concentration in the presence of minimal application of systemic cost as well as maximal suppression of both viral load and pathogenic infections. Therefore, we compatibly opt for an optimal control pair
that is ascribes by the expression
subject to the system of ODEs (1)–(7) and such that
is measurable with
for all
, for
a measurable control set. This is an innovative optimal control theory, which is in line with several varying control theories formulated; see for example.7,9,13,17,19 Next, we verify the existence of the model optimality control for a PMC treatment.
Existence of optimality control strategy
The existence of an optimality control for PMC treatment of a dual HIV–pathogen infections can be proved from the point of.10 where we possibly show that the right sides of equations (1)–(7) are bounded by a linear function of the state and control variables. Also, we show that the integrand of the objective functional (12) is concave on Q and bounded below, which again affirm the compatibility required of the model. Therefore, there exists on the basis of system boundedness of solution, super–solutions of the system
and , satisfying the equation:
,(13)
Where
are half saturation constants on
and P with equation (13) been compactly bounded on a finite time interval. Thus, determining the existence of the optimal control to the model, we invoke theorem 4.1, p. 68–69.10
Theorem 3.1: Given an optimal control system with model equations (1)–(7) and having proposition 2.1, there exists a PMC optimal control
such that
Proof: Taking proceeding from Thm. 4.1.10 we state and show that the following conditions are justified:
- Then control set
is Lebesgue–integrable function on interval
and the corresponding state variable is nonempty.
- The measurable control set Q, is convex and closed.
- The right side (RHS) of the state system is continuous and bounded above by sum of the bounded control and state variables and can by written as a linear function of
, i=1,2 , with coefficients define by proposition 2.1 and on the state variables.
- The integrand of the objective functional is concave on the measurable set Q.
- There exist consistence
and
, such that the integrand
of the objective functional satisfies
.
Then, we at once resort to the result of (.30 Thm. 9.2.1, p.182) for the existence of the state system (1)–(7) with bounded coefficients, which satisfies condition (i). It is obvious that solution here is bounded. It follows by definition that our control set is closed and convex, thus satisfying condition (ii). Since, our state system is bilinear in
, the RHS of (1)–(7) satisfies condition (iii) in the sense of boundedness of solutions. Furthermore, the integrand of the objective functional
is concave on the measurable control set Q. Finally, the completeness of the existence of solution of the optimal control lies in the established fact that
where
depends on the upper bound on V and P; and
since
. This completes the proof.
Optimality control system
Here, relying on the fact that we were able to prove the existence of the model equations, then we can establish the model optimality system. This is achieved by first defining the necessary conditions for an optimal control for periodic multiple treatments under dual infectious variables. Then, for equation (12), the penalty term on the constraints of the objective functional is the Hamiltonian arguments define by the Lagrangian:
=
Where
are the penalty multipliers satisfying
at the optimal
and
at the optimal
. The optimal control
to be determined leads to the following theorem.
Theorem 3.2: Let
be the given optimal control to be determine, such that, if the system
and
are the solutions for the corresponding state variables (1)–( 7), then there exists adjoint variables
, i=1, 2... 7 satisfying
and having
as transversality conditions. Moreover,
and
.
Proof: The fact that the adjoint equations and transversality conditions as stated by the theorem are standard results from Pontryagin’s maximum principle.20,31 we obtain the adjoint system by differentiating the given Lagrangian with respect to state variables
and M as follows:
(14)
.
This gives the optimality equations of the system as:
at
at
.
Hence, we obtain the optimality control
and
as
(15)
(16)
It follows from the boundedness on the controls that
.
Compatibly, we rewrite
as:
.
The corresponding expression for
is derived as:
and having it compact form as:
So, we have shown that the optimality control system is defined by the state system couple with adjoint system and the initial transversality conditions together with properties of the optimal control for PMC treatment deduced as:
(17)
(18)
Therefore, a complete optimality control system is thus, derive by the substitution of (17) and (18) into the original model equations (1)–( 7) and (14) of the adjoint variables i.e.
with
,
,
and
.
Uniqueness of optimality control system
We complete this section with a simple proof of the uniqueness of solution of the optimality system for a small time interval. Achieving this, we explore the following theorem, which takes it leap from the lemma below.
Lemma 3.1: The function
is Lipschitz continuous in z where
are some fixed positive constants.
Theorem 3.3. Let time interval
be sufficiently small, then bounded solutions of the optimality system are unique.
Proof: Given that
and
are two solutions of our optimality system (19). Then, the value for each of the solutions can be define by letting
and
where
is chosen. Furthermore, we let
and
.
Then, we substitute
and all corresponding terms into first ODE of equation (19) and differentiate to obtain
. Similarly, for
and substituting for
into their respective ODEs of equation (19), we obtain
Next, we perform subtraction of state solution
from
,
from
, ….
, from
,
from
,……
, from
. Then, we multiply the solution obtain by appropriate difference of functions and integrate from to . Finally, we proceed to sum all the fourteen integral equations and using estimation approach, we derive the uniqueness of the model solution. Invoking lemma 3.1, we obtain our first result as:
and
The explicit illustration of the estimate, which use
estimate, is given for
as follows:
,
where
and
are constants evaluated by the coefficients and bounds on states and adjoints of the optimality control system. Combining these fourteen estimates yields the following result:
.
holds for all
We thus conclude from the above equation that the inequality
,
where
are functions that depend on the coefficients and bounds of
. Therefore, for any chosen value of
, such that
and
, the expressions
holds. Hence, the solution is unique for sufficiently small time.
We refer readers to models.9,13 for related proofs of uniqueness of optimality control system. Logically, uniqueness for small time interval are conspicuously two–point boundary value problem due to its opposite time orientation and the state equations, which are embedded with initial and final time conditions of the adjoint equations. Furthermore, analysis of Thm. 3.3 shows that if
and
, such that
, infectivity is drastically under control and could be below detectable limits of clinical assay. Intuitively, for
such that
, then endemic infectivity prevails, which could assume global dimension. Next, we investigate the optimality control for periodic multiple chemotherapy treatment.
The scope of this study compels us to the design for a more befitting optimal dual HIV–parasitoid pathogen multiple chemotherapy treatment championed by periodic treatment interruptions. This is to say that we aim at establishing optimal control that best describe treatment schedules under on and off chemotherapy, worthy to be considered as periodic multiple chemotherapy method (PMC – M).
Periodic multiple chemotherapy method
Here, let time assume discrete controls for
and
i.e.
. The implication is that if control vector is 0, treatment is off and if
, treatment is full (i.e. on). Also, if we consider
months i.e.
days, then vector control limit is
months and the set of all such control vectors can be designed as
. Thus, we’re treating an optimal control vector pair (
) that satisfies
and subject to the state system (1)–(7), such that
is defined by (12). The present study varies from other related models.1,13 where drugs time intervals considered, were on daily administration of drugs. This present study considers drugs administration on weekly intervals taking infection set–point as
months.2.
Now, if we let
be the set of elements, then our optimal control pair is satisfied. Furthermore, if a random selection of pair elements is made from this set
, then we can solve the state system using these controls pair. This procedure is repeated for all possible pairs and then choose the value of the objective functional,
, with the smallest cost functional value as the optimal control value pair,
and
. The outcome of this process is obviously cumbersome and thus, lead to complex cost benefit evaluation for state system (1)–(7).
Moreso, retaining the time interval
months, the periodic cost benefit evaluation would be
since each control pair is
vectors, a procedure that is comparatively expensive. So, we could design a more probable iterative process with simpler and shorter computations. Again, we consider 2 weeks segments as against 1 week. This seems more convenient and practicable for the fact that keeping to treatment schedules of highly docile clinical drugs of this magnitude, daily observation is almost not feasible. Therefore, for 2 weeks segments, the complexity for each control pair is reduced to 1*14 from 1*30. The reduced number of iterations is thus
, which still looks large.
Further simplification leads to the consideration of subperiods of such given period i.e.
, a procedure that accounts and lessen the burden of earlier approach but are similar in technique to that by.1,13,23 where only single infection was considered respectively. For easy assimilation, we call this approach, “multiple periodic methods”. A method built upon an optimal PMC control pair
, for a step–wise reducible iteration technique with 2 weeks segments from the first MP method
. The implication is that the magnitude of
and
is
(for weekly segment of 1 month), so the optimal solution is obtain as
iterations as against
iterations from.1 and
iterations from.23 For next period of
, the control pair is
and
, where
is 0 or
.
The explicit procedure is that we take
optimal PMC control pair
and
as the first 4 elements of the controls
and
respectively. Then, we iterate
and
to obtain the next optimal MPC control pair (
,
) over
period, which also give
iterations. The process is repeated for each of the control pair for the time intervals up to
. The PMC control vectors obtained for the entire iterations are
and
, which clearly represents a suboptimal model.
This later technique is an enhanced and extended approach of the models.1,23 which establishes the intermittent (or periodic) application of multiple drugs as a means of controls of drugs severities and maximization of healthy T–lymphocytes cells and macrophages with structured treatment interruption after a prolong chemotherapy administration. From these two models, it is observed that though T–lymphocytes cells and macrophages were maximized, thereby prolonging the life–span of infected patients as well as suppression of viral load, these virions were not completely eliminated. Thus, re–emergence of infection is likely to occur. Therefore, we decisively omits the numerical simulations of our later method, which will also yield an improve result when compared to models.1,23 but definitely not leading to the elimination of viral load and parasitoid–pathogen.
On the other hand, cases abound where due to either lack of availability of drugs for smooth continuity or bored by continuous administration of drugs and in most situations drug side–effects become a constraint for practical optimal continuous application of chemotherapies. Furthermore, chemotherapy observations and alternations require time intervals. This leads to the formulation of optimal control for periodic multiple chemotherapy (PMC) treatment.
Numerical computation: continuous case with no control measures
At this moment, we are obliged to show that optimality system is a two–point boundary value problem. Therefore, we first simulate the state system (1)–(7), using initial conditions as specified by (Table 1 & 2) above. This stage of numerical illustrations shows the interactive behavior of state variables following multiple applications of chemotherapies without optimal control measures on the chemotherapies. Here, we intend to appreciate the model when no controls measures are imposed on treatment factors and thus, having no access to control the systemic cost. The second stage is the methodological application of multiple chemotherapies for the maximization of T–lymphocytes cells and macrophages; and minimization of the systemic cost following the implementation of state adjoint system and transversality conditions. The optimal controls, can then be updated to choice treatment for each iteration using drugs efficacy control formulas (17) and (18) until convergence is attained.
To initiate our simulation, we invoke treatment compactible period from model.17 such that
months (i.e.
days), which accounts for drug validity period. So, for early perturbation of uninfected cells by introduction of dual virions per
blood plasma, and taking initial values as in Tables 1 & 2, we demonstrate as depicted by Figure 2a–g below, the methodological application of multiple chemotherapy with complete zero optimal control measures, satisfying aims (i), (ii) and (iv) of the study. Thus, for a continuous multiple chemotherapy with two treatment (without optimal control measures and penalty conditions,
and
) factors, we observe for: (i)
, which acts on
and
, that Figure 2a exhibits gradual inclination (or maximization) of uninfected T–lymphocytes (CD4+ T cell count) concentration from its initial value of
to an overwhelming
for
months. This outcome is enhanced by the presence of the high immune effectors response
, which is boosted by RTI.
Figure 2b represents the simulation of uninfected macrophages under the same action of RTI. Healthy macrophages is maximized from its initial value of
to an increase volume
, which strongly again, indicate the effect of the presence of immune effectors response.
Furthermore, Figure 2c depicts behavioral infectivity of infected T–lymphocytes (CD4+ T cell count), following the application of RTI in the presence of high immune effectors response. Here, infected T–lymph cells with initial value
, exhibited early growth to a value
, through the first 7 months but declined thereafter to the value
following continuous medication and the presence of high immune effectors response. Infected macrophages cells declined to a negligible value of
in the interval
months. The high concentration of healthy T–lymphocytes in Figure 2a, attest to this latter result. A critical view of Figure 2d, depicts an overwhelming decline of infected macrophages cells, which exhibits undulating negative trend at the 7th month. We as well, observe gradual re–emergence with stability at zero after 30 months of chemotherapy (RTI) in the presence of boosted immune effectors response.
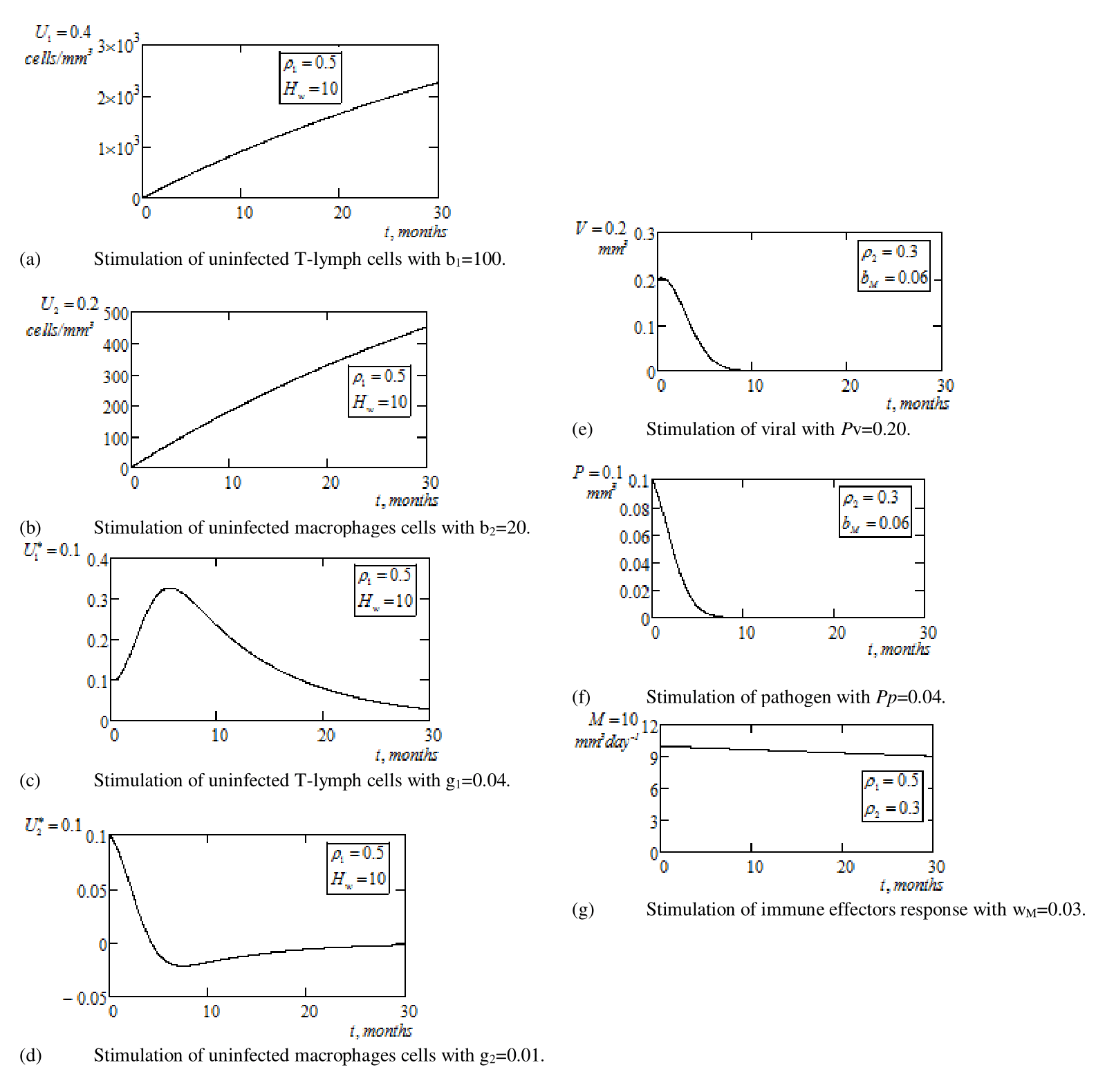
Figure 2 Graphical simulations of continuous optimal multiple chemotherapy treatment without optimal control measures and penalty conditions, and penalty conditions, ρ*1and ρ*2.
On the other hand, the second chemotherapy (PIs), i.e. (ii)
, which predominantly act on virions viral load and parasitoid– pathogen is use to study the biological behavior of these virions in the presence of boosted immune effectors response. Figure 2e depicts eventual elimination of viral load after 9 months of continuous application of treatment measure with high immune effectors response. We see a viral load of initial value of
decline and eliminated to zero after 9 months. Also, the attack of PIs on parasitoid pathogen is depicted by Figure 2f. The outcome is the sharp decline and eventual elimination pathogen after 8 months of continuous PIs chemotherapy.
Finally, Figure 2g represents the biological behavior of immune effectors response, which is boosted by the introduction of multiple chemotherapies (RTI and PIs). Immune effectors response has the essential components, which play critical role of antiviral defense as it attack the virions, thereby increasing the concentration of healthy T–lymphocytes cells and macrophages. The decline of immune effectors response from (
)
is a clear indication of deduction of virions and clearance rate due to attack on both infected cells. In other words, the concentration/increase of immune effectors response in the CD4+ T cells is a function of the amount of virions presence (or virus’s rate of attack on the immune system).
Now, we have seen that the results of the numerical simulations for continuous application of multiple chemotherapies without optimal control measures on treatment factors for a dual HIV–parasitoid pathogen as significantly beneficial to the maximization of both healthy T–lymphocytes and macrophages cells; and boosted immune effectors response, suppresses/reduced viral load and pathogens. The constraints of this result are the inability of the initial state system (1)–(7) to define treatment cost at this level. On this note, we opt to investigate the case for continuous multiple chemotherapy treatment (MCT) with control measures (bounds) on the optimal weight factors of
and
(RTIs and PIs). This approach is hoped to be more rewarding.
Numerical simulation of MCT: continuous case with control measures
Here, observing the state variables and parameter values of Tables 1 & 2, we simulate model (19), which gives us the option to evaluate the cost of treatment. These illustrations account for any possible drug side–effect in cognizance with drug validity period, i.e. drug bounds
. and clinically balance any variations of state variables in the objective functional (12) by the optimal weight factors
and
respectively.
Thus depicted by Figure 3a, we investigate the concentration of uninfected T–lymphocytes cells following the application of optimal control measures on RTIs as indicated by
of equation (17). Furthermore, sustaining Tables 1 & 2 and with the inclusion of the penalty conditions i.e.
, we observe that the concentration of uninfected T–lymphocytes cells experience appreciable increase from
, as was the case when treatment was administered without control measures (Figure 2a). The situation implies that maximization of healthy CD4+ T cells is independent of prolong chemotherapy application.
From Figure 3b, with the application of control measure (as in
), healthy macrophages cells exhibit high concentration at the early months of chemotherapy with stability at
months having value
, before declining to
at 30 months of chemotherapy. The implication is that for maximum restoration of healthy macrophages, chemotherapy most be on short term schedule.
In Figure 3c, we see an enhance minimization/reduction of infected T–lymphocytes cells after initial increase at the early
months. Precisely, infection is seen to decline to
after 30 months of chemotherapy as against
of Figure 2c. The enhance decline over that of Figure 2c, can be attributed to the application of optimal controls on chemotherapies. Also, depicted by Figure 3d, is the drastic reduction of infected macrophages to a value
, following the application of penalty conditions on treatment factors. Here, it is suggested that treatment could be control to avoid early drugs side–effects.
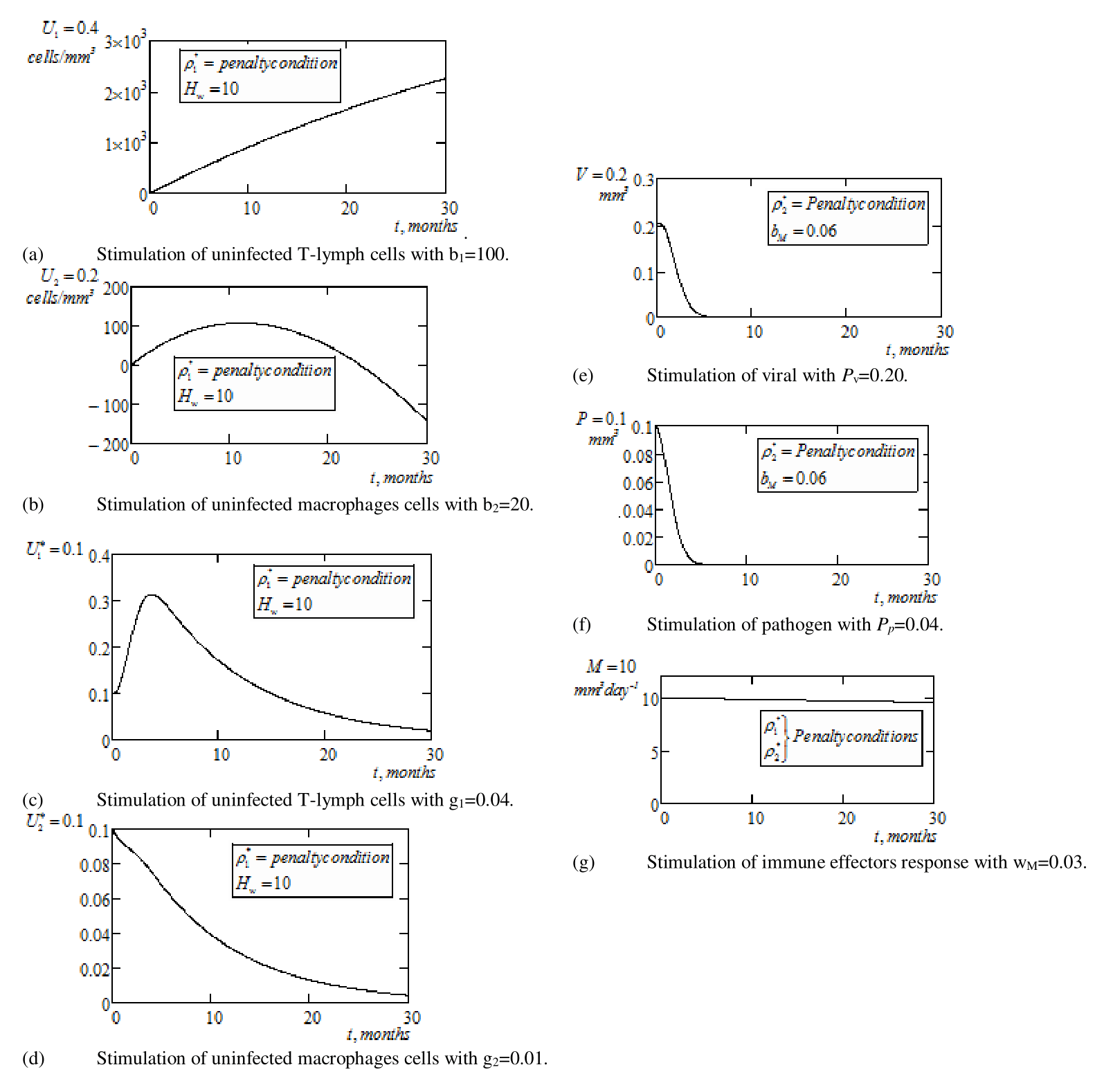
Figure 3 Graphical simulations of continuous optimal multiple chemotherapy treatment with optimal control measures and penalty conditions, ρ*1and ρ*2.
From Figure 3e, with the application of control measure as in
of equation (18), we observe sharp decline of viral load, leading to elimination of viral virus to
after 5 months of continuous clinical application of chemotherapy. Result indicates an improvement in the period of elimination compared to when treatment was administered without penalty conditions on drugs (Figure 2e). In a similar condition, Figure 3f depicts elimination of parasitoid pathogen at the
month of drug application. The time interval for drugs without control measures was 8 months.
The biological behavior of the immune effectors response is represented as in Figure 3g. Here, immune effectors response boosted by the control measures (
), exhibits stability with minimal loss due to clearance rate at the
month i.e.
. This shows that immune effectors response is always maintained at a positive level and it is never eliminated. Again, for brevity graphical representations of the penalty conditions are omitted. Figure 4a & 4b below, simulate the optimal control pair
and
, with defined lower and upper bounds on the optimal weight factors of RTIs and PIs respectively. Here, we see that the most intriguing indication of Figure 4a & 4b are the smooth continuous MCT–like characteristics of the optimal dynamics. Precisely, Figure 4a shows that with high optimal weight factor on RTIs, balanced by low upper bound of
, the toxicity of the drug lies between
. Similarly, PIs with less optimal weight factor and having higher upper bound of
, exhibits drug toxicity in the interval
.
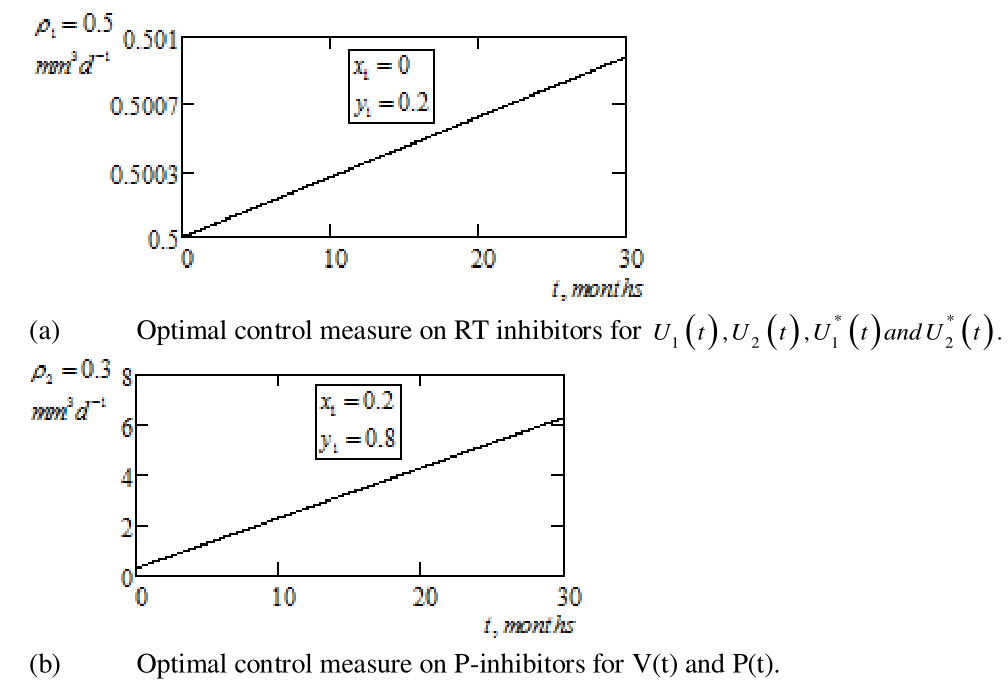
Figure 4 Graphical simulations of optimal control pair for continuous multiple chemotherapy treatment with penalty conditions,
and
.




