Journal of
eISSN: 2473-0831


Research Article Volume 2 Issue 5
Correspondence: Upendra Bhatnagar, Department of Pharmacology & Toxicology, Zydus Research Centre, Cadila Healthcare Ltd, Sarkhej-Bavla N.H. No. 8A, Moraiya, Ahmedabad-382213, Gujarat, India, Tel +912717 665555, Fax +912717 665355
Received: May 17, 2016 | Published: July 7, 2016
Citation: Shah C, Patel J, Rajwadi V, Gupta G, Ranvir RK, et al. (2016) Non-Clinical Safety Assessment of Single and Repeated Administration of Hepatitis B (rDNA) Vaccine. J Anal Pharm Res 2(5): 00035. DOI: 10.15406/japlr.2016.02.00035
Hepatitis B infection is an important public health concern all over the world. As no specific treatment is available, greatest emphasis is placed on prevention through immunization. A recently developed Hepatitis B (rDNA) vaccine, containing purified surface antigen of virus like particles derived from culturing genetically engineered Hansenula polymorpha yeast cells having the Hepatitis B surface antigen gene of Hepatitis B virus was evaluated to establish potential safety and tolerability of the Hepatitis B (rDNA) vaccine formulation. Non clinical safety assessment was evaluated as single dose studies in mice and rats and a repeat dose study in rats. No signs of acute toxicity were noted in mice and rats through clinical signs and gross necropsy examination. The local reactions observed in both the species at the injection site were attributed to the property of an adjuvanted vaccine. Similarly, the absence of systemic toxicity was evaluated after repeated administrations in the rat. In repeated dose study, additionally histiocytosis and/or lymphoid hyperplasia at femoral lymph node were observed. The incidences of such local effects were considered as innate immune response generated against the vaccine antigens and enhanced by the adjuvants. The vaccine was shown to be well tolerated without any obvious signs of adverse systemic toxicity, with findings largely attributable to the adjuvant used. The immunogenicity profile showed measurable antibody titer. Therefore, in these non-clinical models, the single and repeated dose administrations of Hepatitis B (rDNA) vaccine were considered as well tolerated up to absolute human dose (1 ml).
Keywords: Immunogenicity, Intramuscular, Antigens, Adjuvants, Toxicity profile
°C, degree celsius; EDTA, ethylenediaminetetra-acetic acid; IAEC, Institutional ANIMAL ETHICS COMMITTEE; ICR, intelligent character recognition; ID, Identification; IVC: individually ventilated cage; Ltd, Limited; µg: micro gram; mL, milliliter; OECD, ORGANIZATION FOR ECONOMIC CO-OPERATION AND DEVELOPMENT; %, percentage; rDNA, recombinant deoxyribonucleic acid; USA, United State of America; UV, ultraviolet
Hepatitis B virus (HBV) infection is an important public health problem all over the world. It is reported as a serious and common infectious disease of the liver, affecting millions of people throughout the world.1–5 In addition, HBV carriers can transmit the disease for many years.4–6 There is no specific treatment available for the disease and hence care is aimed at maintaining required comfort, nutritional balance and prevention rather than cure. A recently developed Hepatitis B vaccine by Cadila Healthcare Ltd. (CHL) was subjected to battery of nonclinical studies in accordance with the Schedule Y requirements of Drugs and Cosmetics Rules act 2005, Government of India7 and guidelines of European Medicines Agency,8 World Health Organization9 for supporting its clinical development and registration process. In this article, four acute and one sub-chronic repeated dose studies in rodents have been discussed.
Study outline
All toxicity studies have been performed in accordance with the OECD Principles of Good Laboratory Practice. The experimental outline for these studies is based on global regulatory guidelines.7–9 In acute studies, single dose of Hepatitis B (rDNA) vaccine formulation was administered by intramuscular route using multiple sites and intraperitoneal route, at a dose of 0.5 and 1.0 mL/animal in mice and 1.0 and 2.0mL/animal in rats to the treatment group, respectively. Each intramuscular site is restricted with the volume of 0.1mL in mice and 0.2mL in rats, respectively. An independent concurrent vehicle control group was maintained to receive placebo alone in all acute studies to differentiate the effects of placebo from vaccine formulation. All animals were observed for a period of 14 days post dosing for signs of toxicity, mortality, changes in body weight and gross pathological examination. 5 animals/sex/group were included in all four acute studies.
In repeated dose study, the formulation was injected intramuscularly once in every two weeks at three dose levels (low, mid and high) to Wistar rats for a period of 3 months (on day 0, 14, 28, 42, 56, 70 and 84; so a total of seven injection). Here, there was an independent vehicle control group to receive placebo alone and negative (untreated) control group to differentiate the effects of placebo and vaccine formulation. In addition, recovery groups were maintained at high dose along with concurrent vehicle control and negative control groups for a minimum period of one month post treatment to observe the reversibility, persistence, or delayed occurrence of any adverse effects. The dose volume was selected as per the recommended animal welfare guidelines.10 Except for treatment with test item, animals in the vehicle control group were handled in an identical manner to those in the test item groups. The vaccine formulation was injected to each animal using a suitable needle fitted with a graduated syringe. During the experimental period, animals were observed for signs of toxicity, gravimetric changes, clinical, pathological and histopathological changes, immunogenicity and local tolerance effects of the vaccine formulation. 10 animals/sex/group were included in repeat dose study.
Control and test items
Hepatitis B vaccine is produced by recombinant DNA technology, where Hansunela Polymorpha cells containing gene for Hepatitis B surface antigen are grown to achieve maximum growth and then lysed to recover recombinant protein. It is then further purified and formulated with adjuvant system containing immune stimulant alum hydroxide salts. The control and test items were ready-to-use formulations: Placebo and Hepatitis B (rDNA) vaccine, supplied by Vaccine Technology Centre, Cadila Healthcare Ltd. The vaccine and placebo were stored at 2-80C. Each 1.0mL of the vaccine formulation contained 27.5µg of Purified Hepatitis B surface antigen.
Animal husbandry
The studies were designed to use minimum number of animals to meet scientific objectives, the goals and considerations of applicable regulatory requirements.11,12 Healthy young (6-8 weeks) adult Wistar rats and ICR mice of approved IAEC protocols were obtained from Animal Research Facility of Zydus Research Centre and were acclimatized for a minimum period of five days. A thorough detailed clinical examination was performed before randomization. Animals were ranked by body weight and assigned to different experimental groups using a computerized randomization assignment. Animals with body weight not within the range of ± 20% of the mean body weight of each sex were not selected for the study. Mice in groups of five and rats in groups of three to four per sex were housed in clean, sterilized solid floor IVC cages covered with stainless steel grill top having provision for keeping pellet feed and a water bottle. Autoclaved corn cob was used as bedding material. Cages were placed on stainless steel racks and changed at least twice a week or whenever required. Analysis of corn cob did not reveal any contaminants that could reasonably affect the purpose or integrity of the study. Each day, the floor of the experimental room was swept and all worktops and floor were moped with disinfectant solution. All animals were housed within the control environment having temperature range of 25 ± 3°C and relative humidity of 30-70%. The room was illuminated by 12-h light/dark cycles. The experimental animals were provided with UV treated Teklad global diet supplied by Harlan Laboratory, U.S.A. Filtered drinking water (Reverse osmosis water filter system followed by UV treatment) was provided ad libitum. Analysis of feed and water did not reveal any contaminants that could reasonably affect the purpose or integrity of the study. In each cage, animals were identified with individual tail marking and cages identified with cage label indicating the cage and study number, study type, test item code, strain and species, group number and dose, number of animals, animal ID & sex, approved IAEC protocol number, feed type, experiment start and end date and name of the study director.
Parameters
Morbidity, mortality, clinical signs and detailed clinical examination: All experimental animals were checked for morbidity, mortality and injury twice a daily throughout the experimental period in all studies.
In acute studies, clinical signs were recorded in terms of time of onset, duration, severity and reversals of toxic effects on the day of dosing at approximately 30 minutes and 3 time points post dosing. From next day onwards, all animals were observed once a day for signs of toxicity till the end of 14 days observation period. In repeated dose study, all rats from main and recovery groups were observed carefully at least once a day for visible signs of reaction to the treatment. All rats were also observed for detailed clinical examination once before the start of the treatment and thereafter once weekly during the experimental period.
Body weight and feed consumption: In acute studies, body weight was recorded on the day of dosing (prior to dosing), day 3, day 7 and day 14 (post dosing) during the experimental period. While in repeated dose, body weight was recorded on the day of dosing (prior to dosing) and thereafter, weekly till the end of treatment and recovery period to determine the body weight changes. Feed consumption was determined on weekly basis throughout the treatment period till experiment completion.
Ophthalmoscopy & neuro behavioral observations: In repeated dose study. ophthalmic examination was performed on all rats once prior to the commencement of treatment and shortly before each scheduled necropsy, i.e. near the end of treatment and recovery periods from vehicle control, negative control and high dose group.A Neurobehavioral observation (Functional Observation Battery) was performed for vehicle control, negative control and high dose group (6rats/sex/group) during mid-phase of treatment period and shortly before scheduled necropsy.
Immunogenicity: In repeated dose study, except negative control group, blood samples from all groups were collected from retro-orbital plexus under mild anesthesia once during pre-treatment and at the end of treatment and recovery periods to analyze the samples for antibody mediated responses and to correlate with toxicological findings.
Clinical pathology: In repeat dose study, all clinical pathology parameters of main groups were determined after first and last day of injection (within 3 days) and of recovery groups at the end of recovery period. All rats were fasted overnight (with ad libitum water). Blood samples were drawn from retro-orbital plexus under mild anesthesia and urine samples (6 rats/sex/group) were collected via metabolic cage for analysis.
Hematology: The following hematological determinations from whole blood collected in micro centrifuge tubes containing EDTA (2% w/v solution) were carried out using hematology analyzer (Cell-dyn-3700, USA):
|
White blood cell count (WBC) |
Meancell volume (MCV) |
|
Absolute differential leukocyte count |
Mean cell hemoglobin (MCH) |
|
Red blood cell count (RBC) |
Mean cell hemoglobin concentration (MCHC) |
|
Hemoglobin (HGB) |
Platelet count (PLT) |
|
Hematocrit (HCT) |
Reticulocyte count (RETIC) |
Coagulation: Whole blood was collected, in micro centrifuge tubes containing sodium citrate (3.8% w/v solution), from all surviving rats for determination of the following parameters using coagulation analyzer (Coag-uno, Germany analyzer):
|
Prothrombin time (PT) |
|
Activated partial thromboplastin time (APTT) |
Clinical chemistry
Whole blood was collected and processed to serum for determination of the following clinical chemistry analytes using biochemical (Daytona, U.K.) and electrolyte analyzers (Instalyte-plus, USA):
|
Glucose (GLU) |
Globulin (calculated) (GLB) |
|
Total Cholesterol (TCHO) |
Albumin/Globulin ratio (calculated) (A/G) |
|
Triglycerides (TG) |
Creatinine (CREAT) |
|
Alkaline phosphatase (ALP) |
Urea |
|
Alanine aminotransferase (ALT) |
Calcium (Ca) |
|
Aspartate aminotransferase (AST) |
Inorganicphosphorus (Phos) |
|
Total bilirubin (TBL) |
Sodium (Na) |
|
Total Protein (TP) |
Potassium (K) |
|
Albumin (ALB) |
- |
Urinalysis: Urine was collected via metabolic cages and following parameters were evaluated for examination.
Physical Examination: Volume, Color and appearance
Chemical Examination: Glucose, Protein, Ketones, Bilirubin, Urobilinogen, Blood, Leukocyte, Specific gravity and pH.
Microscopy of sediment: Crystals, Epithelial cells, Cast, Erythrocytes, Leukocytes and Microorganisms/parasites.
Necropsy, tissue processing and examination: At scheduled terminal necropsy, animals were euthanized by CO2 asphyxiation followed by exsanguination. For acute studies, necropsy was performed on all animals from treatment and control groups. At necropsy, the animals were examined visually for external abnormalities. The abdominal, thoracic and cranial cavities and their contents were examined. Gross lesions affected organs/injectionsite were collected and subjected to histopathological evaluation. Tissue samples were collected and placed in 10% neutral buffered formalin until processing unless specified.
In repeated dose study, the rats were examined grossly for external abnormalities including palpable masses. The abdominal, thoracic, and cranial cavities and their contents were examined for abnormalities and the standard selection of whole organs (organ pairs) and tissues were weighed prior to fixation. Tissue samples were collected and placed in 10% neutral buffered formalin. Histopathological evaluation was carried out for vehicle control and high dose groups in both the sexes initially. Later the injection site and femoral lymph nodes were processed in all the remaining groups including negative control group. In addition, all the gross lesions were processed and subjected to microscopic evaluation irrespective of the groups. Microscopic findings were peer reviewed.
Local tolerance effects: Local tolerance evaluation was conducted as a part of the repeated dose toxicity study. Tolerance determined by occurrence of erythema or edema both visually at the time of clinical sign observation and microscopically at site(s) of injection.
Data compilation and statistical analysis
Statistical analysis was performed using Graph Pad Prism® Software Version 6.01. For acute studies, data was compared between control and treatment groups, to determine significant changes in body weight employing unpaired Student’s t-test. In case of repeated dose, ANOVA (Analysis of Variance) was used for the comparison of different dosage groups with the control group for different parameters. Bartlett’s test for equal variances was performed for each parameter. Post hoc test to analyze data after ANOVA was done using Dunnett’s test. Tests used in analysis of data were done at 5% and 1% level of significance. Unpaired students t-test was used for the comparison of negative control and vehicle control; vehicle control recovery and high dose recovery groups for different parameters, to find if there was any significant difference. Comparison was done on the basis of individual group data.
Types of Comparison
Single dose studies in mice and rats
Hepatitis B (rDNA) vaccine formulation was well tolerated by intramuscular and intraperitoneal route without any mortality in all acute studies. No treatment related adverse clinical signs were observed in intraperitoneal administration in both the species. However, during intramuscular injection, except mild swelling at the site of injection post dosing during initial days due to aluminium based property of vaccine, no other apparent signs of toxicity were noticed till the end of 14 days of observation period in both the species, which was considered as local immune response against vaccine and/or adjuvant. Body weights of vaccine treated animals were comparable with vehicle control treated animals in both the sexes in all single dose studies throughout the experimental period.
Gross pathological examination did not reveal any gross lesion of toxicological significance in vaccine treated groups as compared to concurrent vehicle control groups in both the sexes by intraperitoneal administration in mice and rats. While intramuscular administration reveled white deposits in the muscle at the injection site, which microscopically revealed focal inflammation in both vaccine and vehicle control treated groups. Induced injection site reactions were the sign of immune response generated against the vaccine antigens and enhanced by the adjuvants. Hence, considered as treatment related non-adverse findings.
Repeated dose study in rats
No mortality or apparent clinical signs of toxicity were observed in any group of rats treated with Hepatitis B (rDNA) vaccine up to 1 mL/animal dose during treatment and recovery period in both the sexes. Mild swelling was noticed during daily clinical signs and detailed clinical examination at the site of injection, after each dose administration and was prevalent for few days during the first two months and was prolonged during the 3rd month of treatment period in mid, high and vehicle control groups. In low dose, mild swelling was observed for approximately a week after last dose administration. During recovery period, mild swelling persisted at the site of injection during few days of the first week in both the sexes. This type of clinical sign was considered as local immune response against vaccine and/or adjuvant.
Body weight (Figure 1) and feed intake in all dose groups were normal and comparable to vehicle control rats. No adverse or treatment related changes were noticed in both male and female rats during ophthalmic and neurobehavioral examination at 1.0 mL/animal dose.
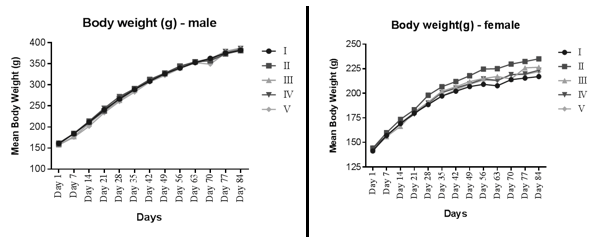
Figure 1 The comparison of body weight of different treatment groups in both the sexes, where I = Vehicle control group (Placebo), II = Low dose (0.25 mL/animal), III = Mid dose (0.5 mL/animal), IV = High dose (1.0 mL/animal) and V = Negative control group.
In clinical pathological investigations (Tables 1–4), most of the findings of statistical variations at the end of treatment and recovery period were minor or non-dose dependent or toxicological insignificant up to the highest dose of 1.0 mL/animal in both the sexes, which could not be correlated with any histopathological alterations. However, some expected treatment related changes were observed on certain clinical pathological parameters like increased WBC, absolute neutrophils, globulin and decreased A/G ratio and were considered to be associated with the expected innate immune response to the adjuvant or vaccine. All other differences observed were considered incidental and hence of no toxicological significance. No adverse changes were found during urinalysis in male and female rats.
|
Matology |
Dose (mL/Animal) / Parameters |
1.0 (Placebo) |
0.25 |
0.5 |
1 |
Negative Control |
|
WBC (103/µL) |
6.47 ± 1.3 |
8.30* ± 1.66 |
7.96 ± 2.16 |
9.40** ± 1.18 |
5.72 ± 1.03 |
|
|
RBC (106/µL) |
8.65 ± 0.37 |
8.55 ± 0.46 |
8.36 ± 0.45 |
8.65 ± 0.3 |
8.6 ± 0.51 |
|
|
HGB (g/dL) |
15.15 ± 0.50 |
15.25 ± 0.42 |
15.05 ± 0.52 |
15.1 ± 0.50 |
15.31 ± 0.77 |
|
|
HCT (%) |
48.00 ± 1.9 |
47.54 ± 1.48 |
47.48 ± 1.83 |
47.78 ± 1.60 |
48.32 ± 2.11 |
|
|
MCV (fL) |
55.51 ± 1.98 |
55.73 ± 2.41 |
56.82 ± 1.87 |
55.25 ± 1.92 |
56.27 ± 1.74 |
|
|
MCH (pg) |
17.54 ± 0.65 |
17.87 ± 0.85 |
18.04 ± 0.64 |
17.45 ± 0.51 |
17.82 ± 0.65 |
|
|
MCHC (g/dL) |
31.61 ± 0.51 |
32.10* ± 0.26 |
31.73 ± 0.21 |
31.60 ± 0.36 |
31.67 ± 0.41 |
|
|
PLT (103/µL) |
692.60 ± 73.39 |
697.00 ± 74.72 |
660.70 ± 81.03 |
728.90 ± 85.49 |
705.60 ± 56.67 |
|
|
ABS. NEUT (103/µL) |
1.989 ± 0.564 |
3.081** ± 0.883 |
3.014 * ± 0.884 |
5.938** ± 0.821 |
1.644 ± 0.538 |
|
|
ABS. LYMPH (103/µL) |
3.796 ± 0.755 |
4.488 ± 0.86 |
4.140 ± 1.573 |
2.682* ± 0.405 |
3.371 ± 0.785 |
|
|
ABS. MONO (103/µL) |
0.425 ± 0.168 |
0.453 ± 0.187 |
0.535 ± 0.145 |
0.473 ± 0.117 |
0.420 ± 0.169 |
|
|
ABS. EOSIN (103/µL) |
0.142 ± 0.033 |
0.178 ± 0.076 |
0.154 ± 0.060 |
0.147 ± 0.062 |
0.127 ± 0.071 |
|
|
ABS. BASO (103/µL) |
0.12 ± 0.1 |
0.094 ± 0.032 |
0.116 ± 0.069 |
0.159 ± 0.113 |
0.160 ± 0.064 |
|
|
PT (Sec) |
11.25 ± 0.48 |
11.14 ± 0.46 |
10.08** ± 0.64 |
10.96 ± 0.42 |
10.97 ± 0.47 |
|
|
APTT (Sec) |
14.83 ± 1.6 |
17.41* ± 1.44 |
18.06** ± 1.75 |
15.35 ± 2.17 |
15.78 ± 2.43 |
|
|
Abs. Reticulocyte (103/µL) |
487.50 ± 121.15 |
613.00 ± 215.84 |
650.70 ± 186.39 |
445.50 ± 108.78 |
540.80 ± 134.92 |
Table 1 Hematology data of male rats
*: Significant at 5% level (p<0.05); **: Significant at 1% level (p<0.01); mL: Milliliter; WBC: White Blood Cells; RBC: Red Blood Cells; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean Cell Volume; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; PLT: Platelet Count; PT: Prothrombin Time; APTT: Activated Partial Thromboplastin Time; ABS: Absolute; LYMPH: Lymphocytes; MONO: Monocytes; EOSIN: Eosinophils; BASO: Basophils; µL: Microliter; g: Gram; %: Percentage; fL: Femtoliters; pg: Picogram; dL: Deciliter; Sec: Seconds
|
Clinical Chemistry |
Dose (mL/Animal) / Parameters |
1.0 (Placebo) |
0.25 |
0.5 |
1 |
Negative Control |
|
GLU (mg/dL) |
93.55 ± 12.70 |
107.63 ± 16.59 |
96.81 ± 8.87 |
80.43 ± 15.45 |
82.73 ± 8.89 |
|
|
TG (mg/dL) |
77.66 ± 18.47 |
62.72 ± 27.79 |
79.17 ± 49.72 |
69.87 ± 20.99 |
107.25 ± 37.55 |
|
|
TCHO (mg/dL) |
54.78 ± 9.38 |
66.93 * ± 9.84 |
62.96 ± 8.16 |
70.12 ** ± 9.58 |
61.28 ± 7.63 |
|
|
AST (U/L) |
114.17 ± 21.50 |
89.62 ** ± 13.92 |
84.93 ** ± 10.57 |
96.60 *± 12.12 |
97.88 * ± 8.69 |
|
|
ALT (U/L) |
38.79 ± 7.56 |
32.42 ± 6.24 |
32.34 ± 4.52 |
34.18 ± 6.55 |
40.91 ± 11.37 |
|
|
ALP (U/L) |
76.50 ± 15.64 |
76.43 ± 16.42 |
76.07 ± 13.87 |
80.18 ± 14.83 |
86.96 ± 17.14 |
|
|
TBL (mg/dL) |
0.22 ± 0.03 |
0.15 ** ± 0.02 |
0.16 ** ± 0.03 |
0.25 ± 0.05 |
0.21 ± 0.05 |
|
|
TP (g/dL) |
5.81 ± 0.27 |
6.31 ** ± 0.19 |
6.27 ** ± 0.17 |
6.03 ± 0.15 |
5.94 ± 0.24 |
|
|
ALB (g/dL) |
3.85 ± 0.15 |
3.69 * ± 0.11 |
3.72 ± 0.06 |
3.87 ± 0.12 |
3.96 ± 0.12 |
|
|
GLB (g/dL) |
1.96 ± 0.16 |
2.62 ** ± 0.11 |
2.55 ** ± 0.14 |
2.16 ** ± 0.11 |
1.98 ± 0.15 |
|
|
UREA (mg/dL) |
34.35 ± 5.73 |
40.48 * ± 2.52 |
36.92 ± 4.95 |
33.97 ± 5.38 |
36.39 ± 4.53 |
|
|
CREAT (mg/dL) |
0.63 ± 0.04 |
0.68 ± 0.04 |
0.62 ± 0.06 |
0.64 ± 0.08 |
0.60 ± 0.08 |
|
|
Ca (mg/dL) |
10.64 ± 0.24 |
10.02 ** ± 0.27 |
10.09 ** ± 0.26 |
10.44 ± 0.35 |
10.62 ± 0.27 |
|
|
PHOS (mg/dL) |
4.35 ± 0.35 |
4.65 ± 0.32 |
4.64 ± 0.41 |
4.51 ± 0.4 |
4.65 ± 0.48 |
|
|
Na+ (mmol/L) |
142.96 ± 0.98 |
142.62 ± 1.35 |
141.99 ± 0.78 |
142.73 ± 1.31 |
142.84 ± 1.09 |
|
|
K+ (mmol/L) |
4.01 ± 0.12 |
3.77 ** ± 0.16 |
3.88 ± 0.24 |
4.16 ± 0.12 |
4.00 ± 0.16 |
|
|
A:G ratio |
1.97 ± 0.13 |
1.41 ** ± 0.06 |
1.46 ** ± 0.08 |
1.80 ** ± 0.11 |
2.01 ± 0.15 |
Table 2 Clinical Chemistry data of male rats
*: Significant at 5% level (p<0.05); **: Significant at 1% level (p<0.01); GLU: Glucose; TG: Triglycerides; TCHO: Total Cholesterol; AST: Aspartate Amino Transferase; ALT: Alanine Amino Transferase; ALP: Alkaline phosphatase; TBL: Total Bilirubin; ALB: Albumin; GLB: Globulin; CREAT: Creatinine; PHOS: Inorganic Phosphorus; Na+: Sodium; K+: Potassium; A:G: Albumin:Globulin; mL: Milliliter; g: Gram; dL: Deciliter; mg = Milligram; mmol = Millimole; L: Liter
|
Hematology |
Dose (mL/Animal) / Parameters |
1.0 (Placebo) |
0.25 |
0.5 |
1 |
Negative Control |
|
WBC (103/µL) |
4.54 ± 0.73 |
5.84 ± 1.34 |
4.92 ± 1.06 |
6.21 ± 1.91 |
4.22 ± 0.77 |
|
|
RBC (106/µL) |
7.85 ± 0.2 |
7.69 ± 0.37 |
7.52 * ± 0.20 |
7.57 ± 0.32 |
7.58 ± 0.43 |
|
|
HGB (g/dL) |
14.47 ± 0.34 |
14.48 ± 0.50 |
14.06 ± 0.36 |
14.11 ± 0.49 |
14.25 ± 1.36 |
|
|
HCT (%) |
45.79 ± 1.03 |
45.77± 1.6 |
44.39 ± 1.51 |
44.42 ± 1.7 |
45.46 ± 4.83 |
|
|
MCV (fL) |
58.36 ± 2.26 |
59.59 ± 1.62 |
59.05 ± 1.38 |
58.7 ± 2.48 |
59.94 ± 2.26 |
|
|
MCH (pg) |
18.44 ± 0.75 |
18.86 ± 0.64 |
18.68 ± 0.40 |
18.65 ± 0.74 |
18.8 ± 0.45 |
|
|
MCHC (g/dL) |
31.61 ± 0.3 |
31.61 ± 0.48 |
31.66 ± 0.47 |
31.77 ± 0.45 |
31.38 ± 0.7 |
|
|
PLT (103/µL) |
736.3 ± 81.62 |
702.9 ± 57.3 |
680.3 ± 107.3 |
763.1 ± 142.43 |
747.9 ± 54.62 |
|
|
ABS. NEUT (103µL) |
0.932 ± 0.250 |
1.395 ± 0.377 |
1.506 * ± 0.312 |
3.542 ** ± 1.417 |
0.867 ± 0.293 |
|
|
ABS. LYMPH (103/µL) |
3.089 ± 0.566 |
3.780 ± 1.09 |
2.859 ± 0.787 |
2.234 * ± 0.518 |
2.886 ± 0.612 |
|
|
ABS. MONO (103/µL) |
0.288 ± 0.046 |
0.398 * ± 0.103 |
0.306 ± 0.079 |
0.218 ± 0.098 |
0.283 ± 0.077 |
|
|
ABS. EOSIN (103/µL) |
0.141 ± 0.044 |
0.169 ± 0.063 |
0.174 ± 0.095 |
0.137 ± 0.036 |
0.110 ± 0.039 |
|
|
ABS. BASO (103/µL) |
0.089 ± 0.021 |
0.096 ± 0.023 |
0.074 ± 0.037 |
0.075 ± 0.06 |
0.074 ± 0.026 |
|
|
PT (Sec) |
9.75 ± 0.44 |
10.14 ± 0.36 |
10.24 * ± 0.28 |
9.4 ± 0.45 |
9.32 ± 0.55 |
|
|
APTT (Sec) |
13.34 ± 0.72 |
15.00 ** ± 1.00 |
14.43 * ± 0.74 |
13.57 ± 0.64 |
13.39 ± 0.81 |
|
|
Abs. Reticulocyte (103/µL) |
848.20 ± 287.01 |
938.20 ± 252.94 |
961.90 ± 112.68 |
887.90 ± 225.72 |
754.50 ± 292.29 |
Table 3 Hematology data of female rats
*: Significant at 5% level (p<0.05); **: Significant at 1% level (p<0.01); mL: Milliliter; WBC: White Blood Cells; RBC: Red Blood Cells; HGB: Hemoglobin; HCT: Hematocrit; MCV: Mean Cell Volume; MCH: Mean Cell Hemoglobin; MCHC: Mean Cell Hemoglobin Concentration; PLT: Platelet Count; PT: Prothrombin Time; APTT: Activated Partial Thromboplastin Time; ABS: Absolute; LYMPH: Lymphocytes; MONO: Monocytes; EOSIN: Eosinophils; BASO: Basophils; µL: Microliter; g: gram; %: Percentage; fL: Femtoliters; pg: Picogram; dL: Deciliter; Sec: seconds
|
Clinical Chemistry |
Dose (mL/Animal) / Parameters |
1.0 (Placebo) |
0.25 |
0.5 |
1 |
Negative Control |
|
GLU (mg/dL) |
104.72 ± 24.18 |
122.52 ± 17.07 |
102.22 ± 16.90 |
115.09 ± 20.29 |
101.09 ± 15.47 |
|
|
TG (mg/dL) |
62.16 ± 18.31 |
46.75 ± 9.57 |
55.33 ± 16.10 |
49.74 ± 14.65 |
59.69 ± 19.99 |
|
|
TCHO (mg/dL) |
54.76 ± 15.39 |
52.66 ± 10.34 |
61.26 ± 15.04 |
55.83 ± 11.35 |
39.09 * ± 12.49 |
|
|
AST (U/L) |
103.6 ± 33.04 |
106.24 ± 29.58 |
108.1 ± 26.67 |
92.17 ± 32.18 |
90.46 ± 14.13 |
|
|
ALT (U/L) |
28.55 ± 4.46 |
28.18 ± 6.64 |
28.13 ± 5.93 |
26.64 ± 7.11 |
26.86 ± 4.3 |
|
|
ALP (U/L) |
31.64 ± 11.8 |
40.57 ± 16.53 |
35.7 ± 9.36 |
31.98 ± 6.67 |
33.66 ± 11.29 |
|
|
TBL (mg/dL) |
0.18 ± 0.03 |
0.17 ± 0.02 |
0.17 ± 0.05 |
0.19 ± 0.04 |
0.19 ± 0.03 |
|
|
TP (g/dL) |
6.68 ± 0.49 |
6.58 ± 0.29 |
6.92 ± 0.33 |
6.66 ± 0.65 |
6.74 ± 0.35 |
|
|
ALB (g/dL) |
4.16 ± 0.28 |
3.94 ± 0.13 |
4.03 ± 0.18 |
4.05 ± 0.37 |
4.25 ± 0.19 |
|
|
GLB (g/dL) |
2.52 ± 0.24 |
2.64 ± 0.18 |
2.89 ** ± 0.28 |
2.61 ± 0.29 |
2.49 ± 0.19 |
|
|
UREA (mg/dL) |
42.25 ± 9.34 |
42.74 ± 5.76 |
49.05 ± 8.48 |
43.5 ± 9.43 |
39.02 ± 7.28 |
|
|
CREAT (mg/dL) |
0.81 ± 0.1 |
0.81 ± 0.12 |
0.82 ± 0.05 |
0.82 ± 0.09 |
0.76 ± 0.06 |
|
|
Ca (mg/dL) |
10.18 ± 0.42 |
10.07 ± 0.37 |
10.38 ± 0.3 |
9.99 ± 0.54 |
9.96 ± 0.42 |
|
|
PHOS (mg/dL) |
3.59 ± 0.58 |
3.92 ± 0.55 |
3.79 ± 0.56 |
4.05 ± 1.19 |
3.61 ± 0.41 |
|
|
Na+ (mmol/L) |
143 ± 1.11 |
142.95 ± 0.96 |
142.64 ± 0.72 |
140.93 ** ± 1.72 |
142.24 ± 1.40 |
|
|
K+ (mmol/L) |
3.72 ± 0.2 |
3.55 ± 0.22 |
3.81 ± 0.18 |
3.70 ± 0.33 |
3.50 ± 0.18 |
|
|
A:G ratio |
1.66 ± 0.1 |
1.50 ** ± 0.07 |
1.41 ** ± 0.14 |
1.56 ** ± 0.07 |
1.71 ± 0.08 |
Table 4 Clinical Chemistry data of female rats
*: Significant at 5% level (p<0.05); **: Significant at 1% level (p<0.01); GLU: Glucose; TG: Triglycerides; TCHO: Total Cholesterol; AST: Aspartate Amino Transferase; ALT: Alanine Amino Transferase; ALP: Alkaline Phosphatase;, TBL: Total Bilirubin; ALB: Albumin; GLB: Globulin; CREAT: Creatinine; PHOS: Inorganic Phosphorus; Na+: Sodium; K+: Potassium; :G: Albumin:Globulin; mL: Milliliter; g: Gram; dL: Deciliter, mg = milligram, mmol = millimole, L = Liter
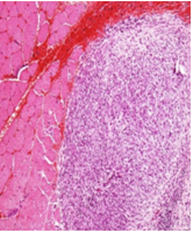
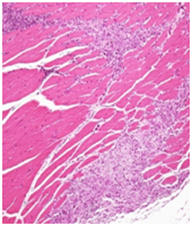
No treatment related adverse changes were noticed in organ weights in both male and female rats. Grossly, no toxicological significant findings were observed. However, treatment related non-adverse findings such as white deposits and/or red discoloration at the injection site were observed in all the treated groups including recovery groups. Microscopically, these lesions were associated with chronic inflammatory and/or hemorrhagic / necrotic changes which were restricted to the injection site only. Similarly, few incidences of enlargement of local lymph node at the injection site (femoral lymph node) were observed across all the treated groups of both the sexes, which microscopically revealed histiocytosis and/or lymphoid hyperplasia. However, this finding was not observed in recovery groups of both the sexes which showing recovery trend at the end of recovery period. All these lesions at the injection site and of local lymph node (Figure 2) were considered as expected treatment related non-adverse findings and were sign of immune response generated against the vaccine antigens and enhanced by the adjuvants. All other gross and histological observations were considered as spontaneous and/or toxicologically insignificant findings.
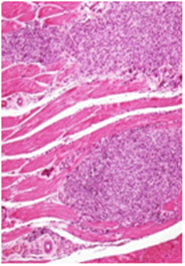
Vehicle Control: Chronic inflammatory changes with necrotic foci at the site of injection in the bundles of muscle fibers. H&E 20X
Low Dose: A compact lesion depicting the chronic inflammatory changes including mild hemorrhagic/necrotic changes in the muscle confined at the site of injection H&E 20X
Mid Dose: Chronic inflammatory changes with necrotic foci at the site of injection in the bundles of muscle fibers similar to those reported in the vehicle groupH&E 20X
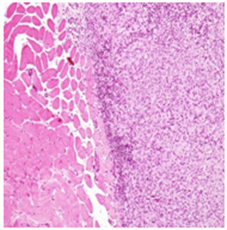
High Dose:A compact lesion depicting the chronic inflammatory changes including mild necrotic changes in the muscle fibers confined at the site of injectionH&E 20X
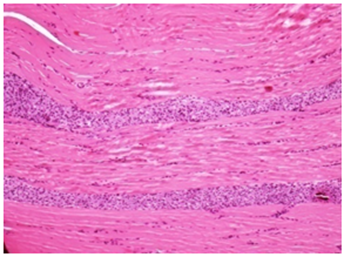
Vehicle Control Recovery: Note the reduced severity of chronic inflammatory changes in the animals after cessation of treatment for 1 months compared main study vehicle control group H&E 20X
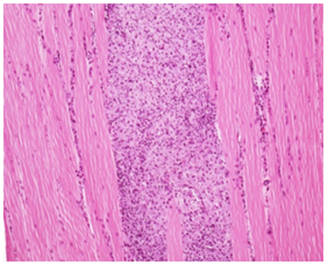
High Dose Recovery: The chronic inflammatory reaction appeared to be in verge of reversion to normalcy & peripheral intact muscle fibers when severity was compared with main treatment groups H&E 20X
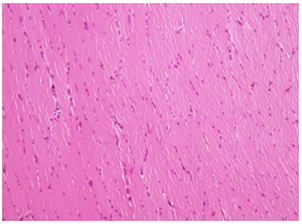
Negative Control: Note the normal architecture of muscle fibers with intact nucleus and sarcolemma muscle at site of injection. H&E 20X
Figure 2A Injection site Lesions.
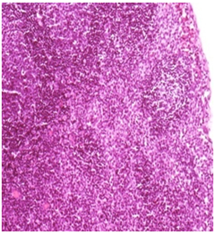
Vehicle Control: Note the uniform distribution of lymphoid element in the femoral lymph nodes adjacent to the site of injection H&E 20X
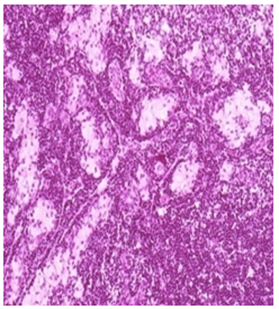
Low Dose: Mild histiocytosis and/or lymphoid hyperplasia was apparent in animal treated with test item as an immune response H&E 20X
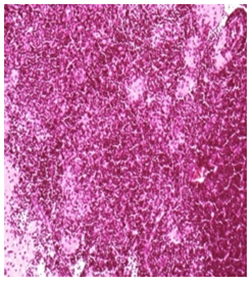
Mid Dose::Mild histiocytosis and/or lymphoid hyperplasia was apparent in animal treated with test item as an immune response H&E 20X
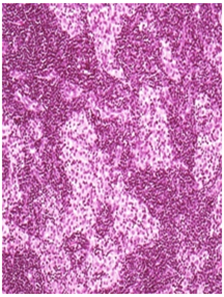
High Dose: Mild histiocytosis and/orlymphoid hyperplasia were apparent in animal treated with test item as an immune response. H&E 20X
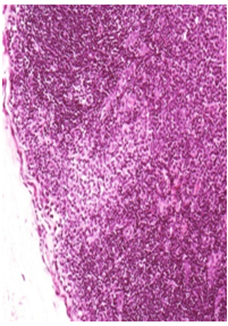
Vehicle Control Recovery: Note the uniform distribution of lymphoid element in the femoral lymph nodes adjacent to the site of injection H&E 20X
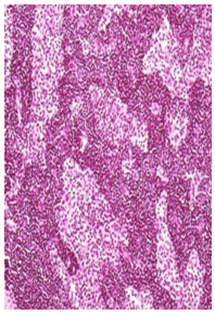
High Dose Recovery: Histiocytosis and/or lymphoid hyperplasia appeared to be reduced in severity during recovery period indicative of reversible nature of lesion H&E 20X
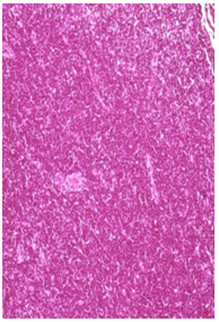
Negative Control: Normal architecture of the femoral lymphnode in animals kept as negative control group. H&E 20X
Figure 2B Femoral Lymphnode Lesions.
Figure 2 Histopathology lesions of repeat dose toxicity in rats (Schematic representation at each dose in random).
The immunogenicity profile showed measurable antibody titer for Hepatitis B (rDNA) vaccine at all vaccine treated dose groups at the end of experimental period in both the sexes.
Dose selected for these studies are based on multiples of absolute human dose (1.0mL). Full human dose (HD) of vaccine was injected in the repeated dose rat study by intended intramuscular route, which was 24 times the equivalent dosage in humans relative to body weight, based on a 6kg body weight of human and 250g rat.
The repeated intramuscular injection of Hepatitis B vaccine in rats at biweekly interval resulted in local inflammation at the injection site within a period of 3 days after the seven injections. This treatment related findings were similar in nature but less pronounced after the seven injection of placebo. Injection site reactions were also seen after a single intramuscular dose of Hepatitis B or placebo in both species. Similarly, other treatment related changes such as increase in the concentration of circulating WBC, absolute neutrophils, globulin together with lower albumin/globulin ratios and microscopic signs of inflammation in the femoral (local) lymph nodes were also noticed in vaccine as well as placebo (less severe; frequent) treated groups after repeated administration.
Inflammatory responses comparable with the findings in these studies were also observed in repeated dose toxicity studies of the MPL-containing human pollen allergy vaccine Pollines Quattro (13). Increase in the concentration of circulating neutrophils, lower albumin/globulin ratios and local inflammation at the injection site were noticed the day after four weekly subcutaneous injections. The role of aluminium adjuvant plays an important part to the acute and chronic inflammatory responses has been very well described.14 Undesired properties of aluminium adjuvant comprise acute and chronic inflammation at the site of injection, its Th2 immune stimulatory capacity, its accumulation besides bio-distribution in the body. Therefore, the inflammatory responses to the Hepatitis B (rDNA) vaccine in the repeated dose study appear primarily associated with adjuvant property. However, the increased severity of some treatment related effects associated with injection site after seven Hepatitis B injections in comparison with seven placebo doses may have reflected inflammatory responses to the vaccine antigen, in addition to aluminium adjuvant.
White deposits, reddish discoloration at injection site, and muscle fiber necrosis and/or some hematology as well as clinical chemistry changes were also observed after one month recovery period, but the effects were generally less frequent and severe than those observed at the end of treatment period suggesting that the process of recovery at the injection site was near complete at the end of recovery. However, the persistence of inflammation at the injection site in some rats may have been due to the residual presence of aluminium salt, as suggested from some other studies on rats and monkeys.15,16
Based on the findings of acute and repeated dose toxicity studies, it is evident that intramuscular and intraperitoneal acute dose of 0.5 and 1.0mL/animal & 1.0 and 2.0mL/animal of Hepatitis B (rDNA) vaccine did not reveal any mortality, unanticipated toxicity and was well tolerated in ICR mice and Wistar rats, respectively. Wistar rats upon repeated intramuscular injection at biweekly interval for 3 months also well tolerated Hepatitis B (rDNA) vaccine formulation without any signs of adverse systemic toxicity up to the dose of 1.0mL per animal (1X of the absolute human dose). Vaccine showed immunogenic response in all vaccine treated groups. All clinical sign observations followed by gross and histopathological examinations and blood analysis (hematology and serum clinical chemistry) revealed changes attributed to the adjuvant based vaccine properties of the tested vaccine. None of these findings had a toxicological significance. Further studies are required to comply with regulatory requirements for the development of Hepatitis B (rDNA) vaccine.
Therefore, it is concluded that Hepatitis B (rDNA) vaccine formulation of Cadila Healthcare Ltd. was well tolerated in all these nonclinical models.
None.
The authors declare there is no conflict of interests.

©2016 Shah, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.