Journal of
eISSN: 2473-0831


Research Article Volume 6 Issue 3
Correspondence: Olumide K Inyang, Centre for Bio computing and Drug Development, Adekunle Ajasin University, Nigeria
Received: September 08, 2017 | Published: October 25, 2017
Citation: Inyang OK, Omotuyi OI, Ogunleye AJ, Eniafe GO, Adewumi B, et al. (2017) Molecular Interaction and Inhibitory Potential of Polyphenol on DNA Repair Pathway in Small Cell Lung Cancer: A Computational Study. J Anal Pharm Res 6(3): 00178. DOI: 10.15406/japlr.2017.06.00178
Poly (ADP) ribose polymerase-1 (parp-1) is a ubiquitous and transient nucleo-protein with supra-function in orchestrating DNA damage repair symphony and has prognostic value in small cell lung cancer (SCLC). Accumulating evidence indicate that the substrate (NAD+) binding catalytic domain (active site) of this protein launches its activation. However, blocking this binding site is the key node in the inhibition of DNA repair pathway, thus signifying its importance in the control of small cell lung cancer progression. Approved drugs for the treatment of disease burden may stop the growth of the tumor cells by blocking some of the enzymes needed for cell growth or by killing the cells, stopping them from dividing via combinatorial efficacy. Clinical tractability of this strategy has been proven to potentially promote undesirable side effect. In the current study, our aim therefore, was to outsource a best in class small molecule inhibitor of PARP-1 from health-friendly source. We therefore, employed computational tools to investigate the therapeutic relevance of compounds from natural source as parp-1 inhibitor via molecular docking approach. 30 phyto-compounds were retrieved from the selected plant, +(-)gallocatechin GC (a polyphenolic compound) showed best binding affinity with the catalytic domain (active site) of parp-1 on comparism to the standard inhibitors. GC interact with the amino acid residue around the 4Ǻ of the catalytic domain (ASN-868, ASN-767, SER-864, GLY-863, HIS-862, TYR-896, PHE-897, TRP-861, ALA-898, SER-904, TYR-907, LYS-903) as evaluated by energy decomposition per residue of GC-parp-1 complex. The result from this investigation thus, project GC as a lead compound which may inhibit PARP-1 as a single therapy in the treatment of small cell lung cancer.
Keywords: enzymes, protein, pathogenesis, nicotinamide, autoparylation
Small cell lung cancer (SCLC), is a highly aggressive pulmonary neuroendocrine neoplasm which represents about 20% of all lung carcinoma1 cases with an ever increasing proliferative index and an unusually strong predilection towards metastasis.2,3 Despite the unceasing basic and clinical research that started about 30 years4 no sustainable improvements have been established in the treatment of this pulmonary insult. Human SCLC is currently ranked as the leading cause of cancer-death worldwide with annual mortality rate. However, several attempts have been geared at understanding the molecular mechanisms that underpin the pathogenesis of human SCLC.
In recent time, SCLC has been characterized by the aberrant expression of number of genes implicated in DNA damage repair pathway such as PARP-1. Wealth of studies has vetted PARP-1 as a critical biochemical therapeutic target and was confirmed by reverse phase protein array (RPPA) method, as one of the overexpressed proteins involved in DNA repair pathway in large SCLC proteomic profiling in cell lines.5 PARP-1 is a chromatin- associated enzyme localized in the nucleus,6-9 programmed to orchestrate DNA damage resolution symphony, especially in the context of base excision repair (BER) during single-strand (SSB), and double-strand break (DSB) repair, this activation process is a survival mechanism. It play single-handed role as an E2F1 co-activator,10-11 this prompt its inhibition may have biphasic action, with direct effects on DNA repair process and in concert with other E2F1-regulated DNA repair proteins. PARP-1 capacity to associate with DNA is established via direct binding to ERK2 which potently stimulates it enzymatic activity and, as a result, increases PARP-1 autoPARylation. PARP-1 activation promotes the ERK2-dependent phosphorylation of a downstream effector (DNA-binding transcription factor Elk1) resulting in an increase in histone acetylation and target gene expression.12 In concert, an NAD+ synthase [nicotinamide mononucleotide adenylyltransferase-1 (NMNAT-1)] associates with PARP1, thus allowing for a proximal source of NAD+ cofactor and increasing PARP-1 activity.
Automodification of PARP-1 transfer signal activation to effector pathways by recruiting repair protein complexes, which may either directly throw in, DNA repair programs or coordinate repair via chromatin unfolding.13 In response to damage, PARP-1 recognises the damage, binds to the injury site and recruit PARP-dependent DNA damage repair proteins to the site such as XRCC1, DNA ligase III, and DNA polymerase β(pol β) to mediate DNA damage repair.14-18 Finally, PARP-1 and the repair complex dissociate from the DNA for a cycling and the single-stranded break is repaired leading to cell survival and the tumour cell growth continues.
Pharmacologic inhibition of PARP-1 by nascent novels in cancer drug arsenal is the strategy against single-stranded breaks (SSB) and double stranded breaks (DSB) repair machinery by the downregulation of key components of the homologous recombination pathway and which eventually abrogate tumor growth5 or making the tumour cell more susceptible to chemotherapy. In presence of parp inhibitor, PARP-1 is still recruited to break site, but can no longer activate parp-dependent DNA repair proteins because it catalytic activity can no longer be powered by NAD+ binding. Therefore, PARP-1 remain bound to the DNA, stalling the replication fork to DNA replication. This stalling drives DSBs formation19-21 and homologous recombination is impaired which eventually leads to cell death and the tumour cell progression is abrogated.
Previous chemotherapeutic strategies targeting PARP-1 solely rely on combinatorial efficacy. Alternatively here, our approach was used based on a singly acting bioactive compounds derived from natural sources to inhibit this candidate protein (PARP-1). Phytopharmaceticals from natural source acting as chemoprotective agent serve as alternative and safer cancer treatments and constitute the major source of current available anti-cancer drugs. In this study, we employ in-silico approaches which provide a high-quality interaction between the ligand and receptor. The bioactive compounds from green tea were screened for by their binding affinities against of PARP-1 catalytic domain to elucidate their anti-tumour effect to overcome tumour cell progression and proliferation by blocking the protein via conformational change which may alter the biological function of the implicated target (PARP-1) in this disease burden. Four lead compounds retrieved from above procedures are then channelled to Lipinski rule of five22 on ADMET (Adsorption, Distribution, Metabolism, Excretion and Toxicity) properties summarized in Figure 1. GC was able to fulfill the rule of five on ADMET properties. Finally we demonstrated that GC may inhibit PARP-1 leading to abrogation of SCLC progression as a safe and single therapy Figure 2.
Target preparation
Molecular docking were employed to the X-ray crystallography structure of human poly ADP-ribosyl polymerase-1 (PARP-1) catalytic domain bound to as isoindolinone inhibitor (FSU) (PDB ID: 4ZZZ) was retrieved from the protein data bank (http://www.rcsb.org). The structure visualized using the molecular graphics program PyMol® intended for the structural visualization of proteins. Water and ligand coordinates were deleted prior to the molecular docking.
Ligand preparation
Two dimensional structure of compounds (phytoligands) in green tea was retrieved from the PubChem® database. Optimized ligand was docked into distinguished model using Ligand Fit theory in the Auto Dock 4.2.
Docking protocol
Molecular docking protocols are widely used for predicting the binding affinities for a number of ligands. In current work, our aim was to examine the possibility of molecular interaction between the experimental bioactivities of the inhibitor under study and compounds from the natural source by their scoring binding conformation. In order to get accurate results, the docking experiment was performed with the default parameters. The protein was treated as a rigid body,23 while the rotatable bonds of the ligands were set to be free. The pre-calculated grid maps at the size set at 60, 60, and 60 A° (x, y, and z) to include all the amino acid residues. The spacing between grid points was 0.375 angstroms. The time to dock one ligand was approximately 1-2 min. Docking using AutoDock/Vina, was performed on a hp workstation (Z800) with an Intel Pentium D processor (3.06 GHz) and Tesla k40 GPU.
ADME screening
ADME (Absorption, Distribution, Metabolism and Excretion) screening for the polyphenolic compounds was done using available online server (http://www.scfbio-iitd.res.in/). ADME screening helps in detecting drug likeliness of compounds. According to Lipinski’s rule of five, the number of rotatable bond, compound molecular weights (MW), calculated logarithm of the partition coefficient between n-octanol and water (CLogP), molar refractivity, number of hydrogen bond acceptors (HBA) and number of hydrogen bond donors (HBD) were used to assess the “drug-likeness” (Lipinski, 2004).
Hydrogen and π-stacking analysis
The two-dimensional representation of molecules is a popular communication medium in chemistry and the associated scientific fields. Computational methods for drawing small molecules with and without manual investigation are well-established and widely spread in terms of numerous software tools. Concerning the planar depiction of molecular complexes, there are considerable less choice. We employ Proteinsplus, an online server (http://proteinplus.zbh.uni-hamburg.de) which automatically generates two-dimensional diagrams of marcromolecular complexes, showing the ligand, interactions, and interacting residues.24,25 We complexed the FSU and GC with the protein separately in pdb format using Pymol and then submitted it on the sever page.
The current study features computational approach to elucidate the molecular interaction, binding mode and inhibitory potential of selected polyphenolic compound of medicinal plant by the procedures displayed in Figure 1 and selected FDA-approved inhibitors on PARP-1 SCLC. Table 1 shows the binding energy and order the order of chemical interaction of natural compound (GC) in comparison to Etoposide, Veliparib and co-crystalized inhibitor (FSU) within the PARP-1 catalytic domain. The binding mode of the compounds and the interacting amino acids around 4Ǻ of human PARP-1 is given in Figure 3a-d while the amino acid residues involved in hydrogen bonding networking and π-stacking interaction between PARP-1 and GC, PARP-1 and co-crystalized inhibitor (FSU) are presented in Figure 5. The docking study was performed using AutoDock 4.2 with PyMol tool whereas Figure 6 displayed the bar chart of the binding energy. The poses snapshot displayed in Figure 4 was done using PyMol. Molecular docking helps in studying the molecular interactions between ligand molecules and target protein macromolecule prior to possible in vitro analysis. Protein structural analysis and ADME assessment were performed on available web server. Human PARP-1 was retrieved from PDB database with PDB ID: 4ZZZ, and use as target for docking simulation. The ligands used in this study were retrieved form Pubchem database in sdf format and prepared for docking simulation. The computed ADME results for the compounds are given in Table 2 and 3D structures of all compounds aforementioned are displayed in Figure 2.
|
Compound |
Binding Energy |
No of H-Bond |
Residues Involved by Hydrogen Bonding |
Amino Acid Residues found Within 4Ǻ |
|
Etoposide |
-11.1 |
4 |
ARG-878, ARG-878, MET-890, TYR-907 |
ASP-770, ASP-768, ILE-872, ARG-878, ILE-879, GLU-763, TYR-889, MET-890, LYS-903, GLU-988, ALA-898, SER-904, PHE-897, TRP-861, HIS-862, GLY-863, TRP-907, SER-864, ILE-872, ARG878, LEU-877, ILE-895 |
|
Veliparib |
-9.2 |
3 |
TRP-861, GLY-863, ALA-898 |
ASN-767, GLU-763, TRY-907, SER-864, SER-904 GLY-863, TRP-861, ALA-898, PHE-897, TYR-896, HIS-862, |
|
Isoindolinone inhibitor(FSU) |
-8.8 |
4 |
HIS-862, GLY-863, ASN-868, SER-904, |
ASN-868, ASN-767, SER-864, GLY-863, HIS-862, TYR-896, PHE-897, TRP-861, ALA-898, SER-904, TYR-907, LYS-903 |
|
GC |
-11.6 |
6 |
PHE-897, SER-904, SER-864, ASN-767, ASP-770, GLN-759 |
ASN-868, ASN-767, GLN-759, SER-864, TYR-907, GLY-863, HIS-862, SER-904, PHE-897, TYR-861, LYS-903, ALA-898,TYR-889 |
Table 1 Showing Etoposide, Veliparib, and GC binding energies obtained from docking with PARP-1
|
Compound |
Molecular Weight |
CLogP |
HBD |
HBA |
Molar Refractivity |
|
Etoposide |
638 |
0.6 |
5 |
13 |
0 |
|
Isoindolinone inhib (FSU) |
246 |
-0.21 |
1 |
3 |
57.57 |
|
GC |
312 |
2.37 |
2 |
7 |
74.72 |
|
Veliparib |
248 |
1.63 |
3 |
1 |
69.42 |
Table 2 ADME result for the established drugs and polyphenol compound on the rule of five formulations
HBA: Hydrogen Bond Acceptor; HBD: Hydrogen Bond Donor; CLogP: The Logarithm of the Partition Coefficient between n-octanol and Water.
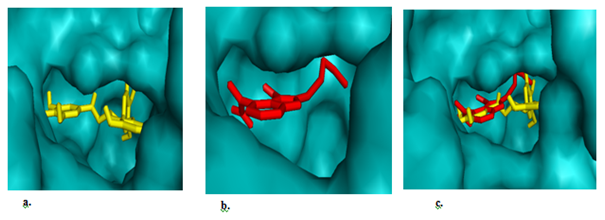
Figure 4 The binding poses of the inhibitors. (a) Stick representation of +(-)gallocatechin (yellow) showing it inhibitory occupancy in the catalytic domain of PARP-1. (b) Stick representation of co-crystalized inhibitor (FSU) (red) in the catalytic domain of PARP-1, (c) a and b showing the inhibitory occupancy of +(-)gallocatechn (yellow) in the catalytic domain over the co-crystalized compound (FSU)red.
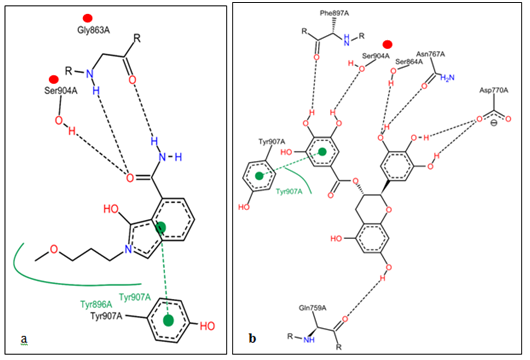
Figure 5 Showing the two-dimensional representation of molecules and their hydrogen bonding network and -stacking. (a) Showing cocrystalized inhibitor (FSU) binding mode of inhibitor of nicotinamide binding pocket as reported via hydrogen bonding network with the amino acid residues (red balls) and -stacking with the aromatic amino acid residue (green dotted line). (b) Showing gallocatechin binding modes of inhibition of nicotinamide binding pocket via hydrogen bonding with SER904 (red ball) and -stacking TYR907 (green dotted line) which has been reported.
Poly (ADP) ribose polymerase-1 belong to PARP family of enzymes. This enzyme has been known for its DNA damage resolution mechanism via poly ADP-ribosylation of proteins involved in DNA repair pathway during DNA damage. It is therefore logical to surmise that inhibiting PARP-1-dependent DSB/SSB repair, represents a sound pharmacological approach.
Here, we identify GC from green tea as a potent inhibitor of PARP-1. Our first strategy was to perform a high throughput PARP-1 based screening to select compounds with favourable binding kinetics. The authenticity of our observations were rightly guided in the light of previously described PARP-1 inhibitors (cocrystalized Isolindolinone Inhib (FSU), Etoposide and Veliparib). The established drugs used in the treatment of extensive stage PARP-1 derived SCLC were docked into the active site (catalytic domain) of PARP-1 with the Gibbs free energy (-8.8Kcal/mol, -11.2 Kcal/mol, -9.2Kcal/mol) respectively. However, docking of the compounds from the natural source against PARP-1 active site using the same grid center coordinate, the compounds that with higher Gibbs free energy above the established drugs were selected, catechin -11.7 Kcal/mol, (−)-epigallocatechin -11.2 Kcal/mol, GC -11.6 Kcal/mol, quercetin -11.5 Kcal/mol, Theaflavin -12.3Kcal/mol. We then deployed Lipinski rule of 5 to all these selected compounds and the established drugs. Excitingly, GC with Gibb’s free energy -11.6Kcal/mol was able to fulfil Lipinski rule of five based on ADMET properties listed in Table 2.
Protein-ligand interaction play a fundamental role in structural-based drug design. In a typical PARP-1 antagonism scenario, clinically relevant inhibition is unambiguously decided by a combo of 12 amino acid residues located within 4Ǻ hydrogen bonding distance in the enzyme’s active site. The interacting amino acid residues within 4Ǻ that participate in stabilizing the protein-ligand complex were (ASN-868, ASN-767, GLN-759, SER-864, TRY-907, GLY-863, HIS-862, SER-904, PHE-897, TYR-861, LYS-903, ALA-898) and they are the main contributors to the stabilization of protein-ligand complex formed by PARP-1 and GC (Table 1 & Figure 3a). To resolve the order of chemical bonds, we studied the binding network of previously described PARP-1 inhibitors and proposed them as benchmarks for efficacious PARP-1 antagonism. It is evident from Figure 5, the polyphenolic compound form hydrogen bonding network with one of the reported critical amino acid residue (SER904), indispensable for the nicotinamide binding pocket of the enzyme26 and hence competitively block the catalytic activity. Hydrogen has a major role in structural stability of many biological molecules and enzyme catalysis. Other molecular interaction between PARP-1 and the phenolic compounds include π–π interaction with the phenolic backbone, hydrophobic and electrostatic attractions. From Figure 5, it is clear that a planarly-oriented π–π stack coupled with a hydrophobic patch which putatively exists between PARP-1 derived TYR907 (an aromatic ring amino acid) and GC aromatic ring. These two are crucial requisite for overcoming possible hydration shell energy and ligand desolvation within the binding pocket.26 From Figure 4c, the polyphenol compound (GC) efficiently occupies the hydrophobic pocket (AD site)27 in an orientation that favours interaction with adjacently positioned but inhibition deciding roles. Most series of PARP-1 inhibitors take the advantage of this spacious pocket to improve solubility, potency and other pharmacologic properties.
Meanwhile, the anti-neoplastic potency of green tea has been clumsily overemphasized with little information about it active pharmaceutical ingredient and target chemistry 28. With PARP-1 being a valid target for anti-cancer drug development, it only makes sense to justify that GC (~4%) may greatly accounts for the plants anti-neoplastic property 29. From Figure 4c, GC fit into a region in the enzyme’s active site where substrate (NAD+) usually binds with a potential of accurately blocking enzyme substrate from assessing the site, powering the enzyme for the cell survival mechanism.
By and large, we have been able to show the binding mode and the order of chemical interaction of GC within the PAPR-1 orthostheric site. Much more importantly, we justifiably predict robust binding kinetics, suitable for PARP-1 antagonism with respect to relevant FDA-approved inhibitors. With the ever increasing morbidity and mortality burdens imposed by SCLC, GC may be, in the nearest future, a single therapy with better inhibitory potency on PARP-1 derived SCLC. Meanwhile, our observations here are still subject to further confirmatory approaches that probe the atomistic, cellular and organism-wide interactions. As well, the polyphenols compounds that are not drug-like (Lipinski’s RO5) can be optimized to explore their pharmacologic efficacy.
None.
None.

©2017 Inyang, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.