Journal of
eISSN: 2473-0831


Research Article Volume 5 Issue 1
Correspondence: Igwe KK, Department of Vet Biochemistry and Pharmacology, Michael Okpara University of Agriculture, Umudike, Tel +234(0)7065297631
Received: June 01, 2017 | Published: June 13, 2017
Citation: Ikpeazu OV, Igwe KK, Otuokere IE (2017) Molecular Geometry Optimization of 5-(Hydroxy methyl) furan-2-carbaldehyde and 3,5-dihydroxy- 6-methyl-2,3-dihydro-4 H -pyran-4-One Active Phytocompounds from Ethanol Leaf Extract of Huru Crepitans and its In Vivo Antimicrobial Activities. J Anal Pharm Res 5(1): 00131. DOI: 10.15406/japlr.2017.05.00131
Geometry optimization of 5-(hydroxymeth yl)furan-2-carbaldehyde and 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (active phytocompounds) from the ethanol leaf extract of Huru crepitans were studied using ArgusLab 4.0.1 software. Minimization was performed with semi-empirical Austin Model 1 (AM1) parameterization. The minimum potential energy was calculated by using geometry convergence function in ArgusLab software. Surfaces were created to visualize the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) and electrostatic potential mapped on electron density surface. The minimum potential energy was calculated for drug receptor interaction through the geometry convergence map. The minimum energy for 5-(hydroxymethyl) furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one were -40.47 au (-25397.71kcal/mol) and -74.97 au ( -47045.04kcal/mol) respectively. At this point these two active compounds of H. crepitans will be more active as an antimicrobial agent. It is possible that H. crepitans will interact with receptor in these conformations. The antimicrobial effects of the phytochemicals in the extracts of Huru crepitans were assessed and evaluation was done against infectious micro organisms; Staphylococcus aureus, Proteus vulgaris, Escherichia coli, Klebsiella pneumonia and Candida albican. The antibacterial activity of the plants was evaluated using Agar gel diffusion method and the minimal inhibitory concentration (MIC) was determined by broth assay method. The extract was tested at varied concentrations of 250mg/ml, 200mg/ml, 100mg/ml, 50mg/ml and 10mg/ml on these five organisms. The Mean Inhibition Zone Diameter (IZD) of Staphylococcus aureus was 4.6±1.10, 8.8±0.32, 12.6±0.89 ,13.4±1.14, 18.6±1.14 compared to the reference drug 10.10± 0.26 at p<0.05. That of Proteus vulgaris was 5.3±0.30, 7.2±0.49, 8.4±0.21,8.4±0.38, 12.4±0.49, 16.6±0.55 compared to the reference drug 7.6±0.54 at p<0.05. Escherichia coli mean Inhibition Zone Diameter (IZD) was 4.6±0.31, 6.1±0.73, 16.0±0.89, 18.5±0.66, 20.7±0.76 compared to the reference drug 16.8±0.48 at (p<0.05). Klebsiella pneumonia was 6.1±0.44, 10.0±0.31, 14.2±0.33, 16.0±0.16, 21.6±1.73 compared to the reference drug 13.3±0.12 (p<0.05). The Mean Inhibition Zone Diameter (IZD) of Candida albicans was 3.8±0.23, 5.2±0.24, 6.5±0.28, 7.4±0.14, 8.0±0.69 compared to the reference drug 00.0± 0.00 (p<0.05). The reference drug had no effect on Candida albicans. This shows that phytochemicals in H. crepitans bark extract are effective against bacteria and fungi therefore could be adopted for pharmacological use.
Keywords:geometry optimization, antimicrobe, huru crepitans, medicinal plant
Applications of phytochemicals in pharmacology are of increasing clinical and commercial importance. Literature publications testify to the growing importance of the discipline and reviews have been published in pharmacognosy. Lists of some clinically used phytochemical agents may be found in most Duck’s work.1 Several researches have worked on the identification and bioactivities of phytochemicals in H. crepitans by GCMS analysis.2 Plants have been used as medicine for curing various ailments and preventing diseases in man and animals. They also play an essential role in religion and development of human culture.3. Analysis of the phytochemicals in medicinal plants helps us to understand the compounds in them that provide us with those medicinal activities for effective health delivery. H. crepitans from the family of Euphorbiaceae (spurge) is an evergreen tree that is native to the tropical regions of the Amazon forest and also the North and South America. Its synonyms are H. strepens Willd, H. brasiliensis Willd and H. senegalensis Baill.4 This plant is also known as sandbox tree, possum wood, jabillo and dynamite tree because of the explosive sound it makes as it splits the capsule.5 H. crepitans can grow up to 200ft (60m)6 and the fruit it produces is in form of a large capsule that can explode, spreading its seeds as far as 160 meters per hour7 or as far as 330ft8 from the tree. The plant is known by possessing many dark pointed sharp spines on its smooth bark. These spines prevent animals from climbing it.9,10 The plant is usually cultivated for shade and the wood used for making furniture, while the milky sap serves as poison for arrows11 and for catching fishes by fishermen.12. H. crepitans is cultivated for medicinal purposes as it is used in treating skin diseases, intestinal worm and srheumatism. Its bark is used to treat leprosy and the leaves used to treat eczema.11. Phytochemicals in plant extracts have been identified using GCMS analysis by different researchers.12-16 thus we use the same analysis to identify the phytochemicals in ethanol extract of H. Crepitans that demonstrated antimicrobial activity as preliminary study and carried out in vivo study on selected microbes.
The purpose of geometry optimization is to determine atomic orientations that are stable. The most stable conformation is one with the least energy. ArgusLab17 is a molecular modeling graphics and drug design program for windows operating system. This research is aimed at confirming the final geometry energy and antimicrobial activities of 5-(hydroxymethyl)furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (active phytocompounds) from the ethanol leaf extract of H. crepitans. The pictorial view of H. crepitansbark and leaf are shown in Figure 1A & 1B respectively. The structures of 5-(hydroxymethyl) furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one as proposed in our previous publication2 are shown in Figure 2A & 2B respectively.
Geometry optimization
Geometry optimization study was performed on a window based computer using ArgusLab and ACD Lab ChemSketch softwares. The chemical structures of 5-(hydroxymethyl) furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one were generated by ArgusLab, minimization was performed with semi-empirical Austin Model 1 (AM1) parameterization. The minimum potential energy was calculated by using geometry convergence function in ArgusLab software. Surfaces created to visualize the highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO) and electrostatic potential mapped on electron density surface. The minimum potential energy was calculated for drug receptor interaction through the geometry convergence map.
Plant materials
Fresh bark of Huru crepitanswas harvested at Umudike town in Abia State, Nigeria. The plant was identified in the Taxonomy section of College of Natural Resources and Environmental Management, Michael Okpara University of Agriculture, Umudike, Nigeria.
Preparation of plant extract
The plant materials of H. crepitans were collected from the wild, shade dried for 10 days and pulverized to powder using mechanical grinder. The plant extract was prepared using Soxhlet method described by.18 Thirty five grams (35g) of powdered sample was introduced into the extraction chamber of the Soxhlet extractor using ethanol as solvent. Temperature was maintained at 75°C throughout the extraction period of 48hrs. At the end of the period, the extract was concentrated using oven at 35°C to obtain dried sample ready for in vivo antimicrobial studies.
Isolation of staphylococcus aureus
Nasal swabs were collected from goat and sample was cultured in mannitol salt media. Golden yellow colonies were observed on the media which is diagnostic for Staphylococcus aureus. Gram staining and catalase and coagulase test was carried out to confirm organism as staphylococcus.
Isolation of E coli, proteus vulgaris, klebsiella pneumonia and candida albicans
The samples were re-isolated from pure stock in the Veterinary Microbiology laboratory in a MacConkey media. Gram staining was done to confirm Gram negative and positive organisms.
Preparation of single-antibiotic discs
100 discs were obtained from punching a No. 1 whatman paper disc and sterilized in a hot air oven at 160°C and 1mlx100 of the re-constituted chloramphenicol solution was pipette into bijou bottle containing the sterile disc and dried in a hot air oven at 60°C.
Antibiotic sensitivity test
An agar well diffusion method was used for the antibiotic sensitivity test. This was done by preparing a nutrient media and the organisms inoculated into the media and incubated for 2hours. Template aseptically was made using a cork borer on one end and the different concentration of extract placed in it. The chloramphenicol disc was placed on the other end of the dish. The plate was incubated overnight and read by measuring the diameter of zone of inhibition for each concentration of H. crepitans bark.
Media preparation
The media used for the antimicrobial sensitivity testing was Muller Hinton agar. It was prepared by weighing out 38g of the powered agar into 100ml of distilled water in a conical flask. This was sterilized in an autoclave at 121°C for 15 minutes, after autoclave, the media was poured into sterile petri dish and allowed to cool and gel.
Determination of antimicrobial activity
The organisms used are Escherichia coli ((2 strains) from the family of Enterobactriaceae and Staphylococcus aureus (2 strains) from the family of Staphylococcus. Stock culture in Vet. Microbiology lab was inoculated into the already prepared Muller Hinton agar. Using a cork borer, well (7mm in diameter and 2.5mm deep) was bored into the inoculated agar and 50μl of each of the extract at a concentration of 1g/ml was delivered into the wells. The plates were incubated 35°C and read after 24hours. The diameter zones of inhibition produced by the extract were measured with a transparent meter rule in mm.
Determination of minimum inhibitory concentration (MIC)
The minimum inhibitory concentration (MIC) is the lowest concentration of antimicrobial extract that inhibited the visible growth of the microorganism after overnight incubation. This was determined by Mueller Hinton agar method.19 To determine the MIC 1.0mL of Mueller Hinton Broth was transferred into 9 test tubes. One (1ml) of the extract at 50mg/ml was pipette into the first tube and mixed properly. One (1ml) was taken from the first test tube into the second test tube and mixed. This was continued up to the 7th tube to give concentrations of 25, 12.5, 6.25, 3.12, 1.56, 0.78 and 0.39mg/ml. The 8thtube was labelled the organism control which contained only the organisms and Mueller Hinton Broth but no extract. The 9th tube was labelled antibiotic control which contained the organism, Mueller Hinton Broth and antibiotic. Volume 0.05ml (50ul) of the organism suspension was transferred into each test tube using a micropipette. The tubes were incubated at 35°C and result read after 24hours. The MIC was the concentration that prevented visible growth of the organism after the period of incubation of 24hours.
Data analysis
Data collected were analyzed using SPSS version 17 software. All values were expressed as the mean value± standard deviation by one way ANOVA for comparisons of the multiple means. It was followed by post hoc test to compare their differences. (p<0.05) was considered statistically significant difference between test and control groups for measured values.
The highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), electrostatic potential mapped on electron density surface, geometry convergence map of 5-(hydroxymethyl) furan-2-carbaldehyde are shown in Figure 3-6 respectively. The highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), electrostatic potential mapped on electron density surface, geometry convergence map of for 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one are presented in Figures 7-10 respectively. The minimum inhibition concentration (MIC) mg/ml of the extract and mean inhibition zone diameter (IZD) mm of different organisms are presented in Figures 11-15.
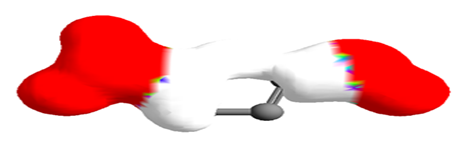
Figure 5 Electrostatic potential mapped on electron density surface for 5-(hydroxymethyl)furan-2-carbaldehyde.

Figure 7 Highest occupied molecular orbital (HOMO) of 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one.
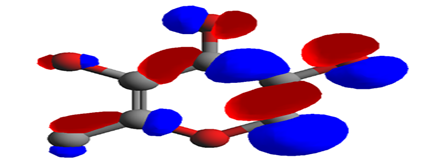
Figure 8 Lowest unoccupied molecular orbital (LUMO) of 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one.
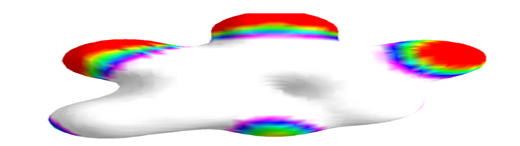
Figure 9 Electrostatic potential mapped onto the electron density surface of 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one.
ArgusLab was used to create mapped surfaces. The electrostatic potential surface was mapped onto the electron density. The electrostatic potential represents the colour while the electron density represents the shape of the molecule. Negative electrostatic potential shows stability for a positive test charge while positive electrostatic potential shows instability for a positive test charge. Thus an electrostatic potential mapped density surface might give information about nucleophilic or electrophilic attack. This enables the Chemist to determine the chemical reactivity of the drug. The red color indicates the most negative regions of the electrostatic potential where a positive test charge would have favorable interaction energy. For 5-(hydroxymethyl) furan-2-carbaldehyde, the negative regions are the COOH and OH functional groups. The ring portion of the molecule with the white color, shows regions of relatively unfavorable energy for the electrostatic potential. For 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one, the negative regions are the OH and C=O functional groups. The methyl-end of the molecule, with the magenta color, shows regions of relatively unfavorable energy for the electrostatic potential.
Highest occupied molecular orbital (HOMO) is the highest energy molecular orbtal that has electron in it. The Lowest unoccupied molecular orbital (LUMO) is the next lowest energy orbital in which an excited electron will occupy. The red regions represent high electron density (negative) while the blue regions indicate low electron density (positive).
The minimum energy for 5-(hydroxymethyl)furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one were -40.47 au (-25397.71kcal/mol) and -74.97 au (-47045.04 kcal/mol) respectively. At this point these two active compounds of H. crepitans will be more active as an antimicrobial agent. It is possible that H. crepitans will interact with receptor in these conformations.
In Figure 11 there was concentration dependent increase in inhibition of the organisms by the extract 4.6±1.10, 8.8±0.32, 12.6±0.89 ,13.4±1.14, 18.6±1.14 as the concentration increased from 10mg/ml to 250mg/ml when compared to the reference drug 10.10± 0.26 (p<0.05). The extract inhibited the growth of Staphylococcus aureus significantly.
Figure 5 showed a concentration dependent increase in inhibition of Proteus vulgarisby the extract 5.3±0.30, 7.2±0.49, 8.4±0.21,8.4±0.38, 12.4±0.49, 16.6±0.55 as the concentration increased from 10mg/ml to 250mg/ml, when compared to the reference drug 7.6±0.54 ( p<0.05). The extract inhibited the growth of the Proteus vulgaris significantly.
From Figure 6 it was deduced that there was concentration dependent increase in inhibition of Escherichia coliby the extract 4.6±0.31, 6.1±0.73, 16.0±0.89, 18.5±0.66, 20.7±0.76 as the concentration of Huru crepitan increased from 10mg/ml to 250mg/ml and compared to the reference drug 16.8±0.48 (p<0.05). The extract inhibited the growth of Escherichia colisignificantly.
From the graph Figure 7, it is shown that there was concentration dependent increase in inhibition of Klebsiella pneumoniaby the extract 6.1±0.44, 10.0±0.31, 14.2±0.33, 16.0±0.16, 21.6±1.73 as the concentration of Huru crepitan increased from 10mg/ml to 250mg/ml and compared to the reference drug 13.3±0.12 (p<0.05). The extract also inhibited the growth of Klebsiella pneumoniasignificantly.
The graph, Figure 8 shows concentration dependent increase in inhibition of Candida albicans by the extract 3.8±0.23, 5.2±0.24, 6.5±0.28, 7.4±0.14, 8.0±0.69. The reference drug had no effect on Candida albicans. There was high inhibition of the growth of Candida albicans by the Hura crepitans bark extract as the concentration of Huru crepitans increased from 10mg/ml to 250mg/ml and compared to the reference drug 00.0±0.00 (p<0.05).
The phytochemicals, 5-(hydroxymethyl) furan-2-carbaldehyde and 3, 5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one in H. crepitans were geometrically optimized. The minimum energy of the phytochemicals were -40.47au (-25397.71kcal/mol) and -74.97 au (-47045.04 kcal/mol) respectively. At this point these two active compounds in H. crepitans will be more active as antimicrobial agent. It is possible that H. crepitans will interact with receptor in these conformations. The in vivo antimicrobial studies confirmed that H. crepitans inhibited Staphylococcus aureus, Proteus vulgaris, Escherichia coli, Klebsiella pneumonia and Candida albican.
We are grateful for the research grant from Abia State Government, Nigeria.
The authors declare no conflicts of interest related to this article.
None.

©2017 Ikpeazu, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.