Journal of
eISSN: 2473-0831


Research Article Volume 12 Issue 1
1Chemistry Department, Haramaya University, Ethiopia
2Chemistry Department, RKSD (PG) College, India
3Chemistry Department, CCS Haryana Agricultural University, India
Correspondence: Om Prakash Yadav, Chemistry Department, CCS Haryana Agricultural University, India
Received: December 29, 2022 | Published: January 13, 2023
Citation: Merera S, Kumar A, Yadav OP. Micellar and intermolecular interaction study of cetyltrimethyl ammonium bromide (CTAB) and Tween-20 mixed aqueous solutions. J Anal Pharm Res. 2023;12(1):13-17. DOI: 10.15406/japlr.2023.12.00418
From surface tension measurements critical micelle concentration (CMC) of aqueous binary mixtures of a cationic surfactant, cetyltrimethylammonium bromide (CTAB) and a nonionic surfactant polyoxyethylene (20) sorbitan monolaurate (Tween-20) have been determined at different mole fractions. Using the CMC value as an input, composition of mixed micelles and the interaction parameter, β, were determined by Rubingh’s theory. Activity coefficient of each surfactant in mixed micelle was less than unity. The observed negative value of the interaction parameter (β) and decrease of Gibb’s free energy indicated synergism in lowering the CMC of the mixed surfactant system and more stability of the mixed micellar system. The observed synergistic effect of mixed surfactant system in lowering as well stabilizing the CMC may be useful in pharmaceuticals for designing efficient targeted drug delivery system, improving mineral floatation process, more efficient petroleum recovery in the tertiary process, and managing effectively the problem of coastal oil spill.
Keywords: pharmaceuticals, CMC, interaction parameter, surfactant, synergistic
Surfactants with lyophobic and lyophilic groups in their molecules are amphipathic in nature and are critical components in pharmaceutical products. These find several uses in pharmaceuticals such as: surfactant self-assembly vehicles for oral and transdermal drug delivery, solubilizing hydrophobic drugs in aqueous media, as components of emulsions, and as agents for improving drug absorption and penetration. Whereas, the non-ionic surfactants are commonly used in pharmaceuticals, the cationic surfactants exhibit antibacterial properties by disrupting bacterial cell membranes. Further, the biosurfactants are employed in vaccines, as anti-adhesive biological coating for biomaterials, in gene therapy and can be incorporated into probiotic preparations to combat urogenetical tract infections and pulmonary immunotherapy.
The physicochemical studies of mixed surfactant systems are of theoretical as well as practical importance1-5. Mixed surfactant systems, due to their better chemical and surface active properties over the individual surfactants, are widely used in pharmaceuticals, detergency, cosmetic, enhanced oil recovery and as flotation agents in metallurgy. Bhattacharjee et al.6 developed and characterized anionic mixed micelles of two biocompatible surfactants, Tween 80 (T-80) and sodium deoxycholate (NaDC), and evaluated their potential in the delivery of doxorubicin hydrochloride (DOX), a cationic anticancer drug. The in-vitro cytotoxicity studies in various cancer cell lines revealed that DOX-loaded micelles have greater in-vitro anticancer activity as compared to DOX solution, indicating their potential in pharmaceutical applications.
Industrial surfactant systems are typically mixtures of different chemical species such as ionic and non-ionic surfactants, electrolytes and other additives to obtain beneficial synergistic effects such as surface tension reduction or to control physicochemical properties of the system such as ionic strength, viscosity and pH7. For choosing an appropriate mixed surfactant system, the knowledge of intermolecular interactions between components is required. Synergistic effects of mixed surfactants has been extensively studied in the light of various theoretical approaches.8-10 Burman et al.9 adopted a systematic approach for studying synergism in mixed surfactant system by keeping the alkyl carbon number same in all surfactant components, as hydrophobic effect is the major driving force of micellization.
The interest in multiphase systems including surfactant solutions is due to their importance in various applications such as in mineral processing, pharmaceuticals, food production, cosmetics, and biomedicine.11-14 Mixtures of different types of surfactants generally exhibit synergism in their properties due to non-ideal mixing effects in aggregates resulting in further lowering of CMC as well as interfacial tension than would be expected in case of unmixed surfactants.15 This has led to interest in developing a quantitative understanding of the behavior of mixed surfactant systems and the same can be exploited in applications such as detergency, enhanced oil recovery,16 mineral floatation,17 pharmaceuticals,6 Sugihara et al.18 reviewed the synergism in micellization as well as in adsorbed film formation upon mixing of nonionic Gemini surfactant with zwitterionic or anionic surfactants. They have discussed the mixed surfactants in terms of interaction parameters, surface excess concentration, partial molecular area, minimum surface Gibbs energy for the evaluation of synergism. Mixed surfactant systems comprising a cationic ester-bonded dimeric surfactant with some monomeric surfactants in aqueous media were investigated by Fatma et al.19 They reported synergism of mixed surfactant systems based on their observed mixed CMC, aggregation number and Stern-Volmer constant using surface tension and fluorescence quenching techniques. Bahareh et al.20 determined CMC in single and mixed surfactant systems, using the UV–Vis Spectroscopic technique. They found that UV irradiation causes the formation of smaller micelles which is of prime concern in membrane technology. Singh & Tyagi21 studied association and aggregation properties of mixed surfactant systems of lauryl alcohol-based bissulfosuccinate anionic gemini surfactants with conventional surfactants in aqueous media using fluorometric technique.
Shiloach & Blankchtein22 analyzed ionic-nonionic mixtures using an ideal solution model. They treated the micelles or the surface layer as a separate phase with composition different from the bulk phase. The theoretical approach of Rubingh,23 based on regular solution theory, accounts for non-ideal mixing of surfactants. A number of molecular thermodynamic theories have also been developed for predicting the property and interaction in binary surfactant system.24,25 These theories were analyzed in terms of different formalisms26 to understand behavior of binary surfactant systems in aqueous solutions based on phase separation model and assuming ideal mixing of surfactants in miceller phase. Matsuda et al.27 studied the origin of interaction between ionic and non-ionic surfactants in adsorbed films and micelles by applying the thermodynamic treatment and studied the effect of ionic head group and counter ion variation. Wiertel-Pochopien et al.28 have studied the synergistic effects in binary surfactant mixtures on foamability (two-phase system) and floatability of quartz (three-phase system) using cationic alkyltrimethylammonium bromides (CnTAB, with n = 8, 12, 16, 18) and n-octanol as the nonionic surfactant. Omer et al.29 have studied interfacial phenomena in the mixed surfactant solutions of sodium di-2-ethylhexylsulfosuccinate (AOT) and hexadecyl benzylammonium chloride (HDBAC).
Cetyltrimethylammonium bromide (CTAB), a quaternary ammonium surfactant, is one of the components of the topical antiseptic, cetrimide - an effective antiseptic against bacteria and fungi. CTAB is one of the main components of the buffer used for the extraction of DNA and has been used in the templated synthesis of nanoparticles involved in cosmetics formulation. Tween-20, a stable and non-toxic surfactant, is used as a detergent and emulsifier in a number of domestic, scientific, and pharmacological applications. It is also used as a washing agent in immunoassays and as a solubilizing agent of membrane proteins and also as a wetting agent in the elastomer industry. In the present work, through surface tension measurements, the nature of intermolecular interactions within mixed micelles comprising a cationic surfactant (CTAB) and a non-ionic surfactant (Tween-20), in aqueous medium, have been investigated.
Cetyltrimethylammonium bromide (CTAB) (C19H42BrN; 98%; MW: 364.45 g/mol), and polyoxyethylene (20) sorbitan monolaurate (Tween-20) (C58H114O26; 98%, MW=1227.54 g/mol) were procured from Acros Organic Ltd. (USA). Chemical structures of CTAB and Tween-20 are shown in Figure 1.
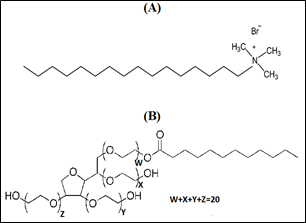
Figure 1 Chemical structure of surfactants: (A): Cetyltrimethylammonium bromide (CTAB), and (B): Polyoxyethylene (20) sorbitan monolaurate (Tween-20).
Methods
Surface tension measurement: Surface tension of CTAB + Tween-20 aqueous solutions were measured at 298.15 K by drop-weight method using a stalagmometer (Allied Plating Supply, Inc.) (Figure 2), and the same was calibrated using surface tensions of some pure liquids: benzene, ethanol, glacial acetic acid, toluene and water as standards.
Plots of surface tension against log [CTAB] for CTAB + Tween-20 mixed surfactant aqueous solutions at 298.15 K as a function of varying mole fractions of CTAB (αCTAB) are presented in figure 3. The critical micelle concentration (CMC) values at different mole fractions of CTAB obtained from the inflection point of respective plots, are recorded in Table 1.
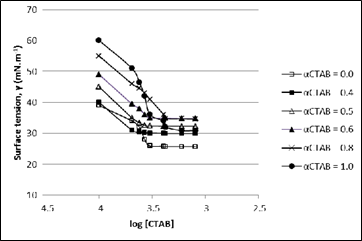
Figure 3 Plots of surface tension, γ (mN.m-1) as a function of log [CTAB] for CTAB+Tween-20 mixed surfactant aqueous solutions at 298.15 K and at varying mole fractions of CTAB (αCTAB).
Mole Fraction of CTAB (αCTAB) |
Experimental CMC(mM) |
0.0 |
0.19 |
0.4 |
0.24 |
0.5 |
0.27 |
0.6 |
0.30 |
0.8 |
0.43 |
1.0 |
0.89 |
Table 1 Experimental critical micelle concentration (CMC) of CTAB+Tween-20 aqueous solutions at 298.15 K at different mole fractions (α) of CTAB determined using surface tension method
Comparison of ideal mixed CMC and experimental mixed CMC
Ideal mixed CMC of studied binary surfactant system was calculated from the pseudo-phase thermodynamic model30 using the relation:
1/C12 = α1/C1 + (1- α1)/C2 (1)
where C12 is the ideal mixing CMC of the studied binary surfactant mixture in aqueous solution, α1 is the mole fraction of surfactant 1(i.e. CTAB) in the bulk; C1 and C2 are the CMC values of surfactant 1 and surfactant 2 (i.e. Tween-20), respectively. Ideal mixed CMC and experimental mixed CMC values for CTAB + Tween-20 aqueous solutions at different mole fractions of CTAB are given in table-2, and their corresponding plots are presented in Figure 4. Experimental mixed CMC are lower than the corresponding ideal mixed CMC at all the studied mole fractions of CTAB for CTAB+ Tween-20 aqueous solutions. This may be due to the screening of the head group charge of the cationic surfactant (CTAB) by the non-ionic surfactant (Tween-20) molecules causing diminished ion-ion head group repulsion of CTAB thus resulting in the lowering of the observed mixed CMC. Such deviation of experimentally observed mixed CMC from the ideal mixed CMC indicates non-ideal behavior of the examined surfactant mixture and the synergetic interaction of unlike surfactant molecules in the micellar phase.
|
αCTAB* |
Ideal mixed CMC (mM) |
Experimental Mixed CMC (mM) |
|
0.0 |
0.19 |
0.19 |
|
0.4 |
0.29 |
0.24 |
|
0.5 |
0.32 |
0.27 |
|
0.6 |
0.37 |
0.30 |
|
0.8 |
0.52 |
0.43 |
|
1.0 |
0.89 |
0.89 |
Table 2 Ideal mixed CMC and experimental mixed CMC values for CTAB + Tween-20 aqueous solutions at varying CTAB mole fraction (αCTAB).
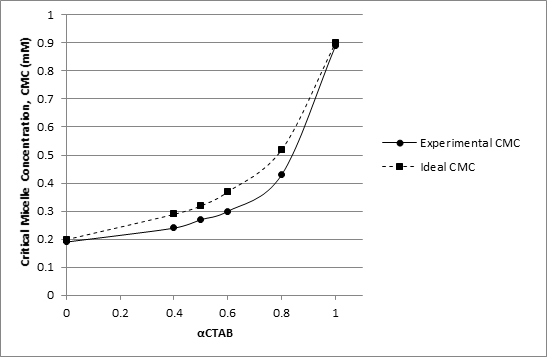
Figure 4 Plots of ideal mixed CMC and experimental mixed CMC as a function of mole fraction of CTAB for CTAB + Tween-20 aqueous solutions.
Determination of micellar composition
According to the Rubingh’s regular solution theory of mixed micelles,23 the CMC of mixed surfactant system can be obtained using the relation:
1/C12 = α1/γ1C1 + (1- α1)/ γ2C2 (2)
where γ1 and γ2 are activity coefficients of the surfactants 1 and 2, respectively; other parameters are similar as described under equation (1). Micellar composition of mixed surfactant system can be determined from the equation:
[(X1)2.ln (α1C12/X1C1)] / [(1-X1)2.ln {(1- α1)C12/(1-X1)C2}] = 1 (3)
where X1 is the mole fraction of surfactant 1 (here CTAB) in the mixed micelle, C1 and C2 are the CMC values of pure surfactants 1 and 2, respectively, C12 is the mixed CMC and “α1” is the mole fraction of surfactant 1 in the bulk of the binary surfactant aqueous solution. The X1 values obtained, iteratively, from equation (3) are given in Table 3. The ideal mole fraction value of CTAB (X1ideal) can be calculated from the equation:
X1ideal = α1.C2 / (α1.C2 + α2.C1) (4)
The observed ideal mole fraction of CTAB (X1ideal) in the micelles of CTAB + Tween-20 aqueous solutions are lower than X1 as seen in Table 3. This indicates higher contribution of CTAB component to the mixed micelle and non-ideal synergistic interaction in mixed micellar state.
|
Mole fractions (α1) of CTAB in bulk |
Mole fraction (X1) of CTAB in mixed micelle |
Ideal mole fraction (X1ideal) of CTAB in mixed micelle |
Interaction parameter (β) |
|
0.0 |
0.000 |
0.000 |
- |
|
0.4 |
0.230 |
0.125 |
-1.29 |
|
0.5 |
0.271 |
0.176 |
-1.11 |
|
0.6 |
0.328 |
0.243 |
-1.48 |
|
0.8 |
0.476 |
0..461 |
-0.79 |
|
1.0 |
1.000 |
1.000 |
- |
Table 3 Bulk mole fraction (α1), micellar mole fraction (X1), and ideal micellar mole fraction (X1ideal) of CTAB, and interaction parameter (β) in mixed micelle
As proposed by Maeda,24 apart from electrostatic interactions, the chain-chain interactions play a major role in the formation of mixed micelle especially for the nonsimilar chain lengths. Since the hydrocarbon chains of CTAB and Tween-20 surfactants are different from each other, a non-ideal behavior is expected. Mole fraction of CTAB within micelle is lower compared to its mole fractions in the bulk due to a stronger ion-dipole interaction between ionic and non-ionic groups31 in the closer environment inside micelle.
Interaction parameter
Interaction Parameter is the indicator of degree of interaction between the unlike component molecules in the mixed micellar state relative to interaction between like molecules before mixing under similar condition. The magnitude of interaction parameter can account for the extent of deviation of the mixed system from ideality and it can be calculated from the following equation:32
β = ln [α1.C12/(X1.C1)] / (1-X1)2 (5)
Where, β is interaction parameter, and all other parameters are as defined under equation (3).
Interaction parameter (β) values for the studied surfactant mixtures are negative (table 3) at the studied mole fractions of CTAB in CTAB+Tween 20 mixtures. This suggests that the interaction between the unlike surfactant components in the mixed micellar phase is less repulsive as compared to interaction between like molecules. Whereas, higher negative value of ‘β’ indicates stronger attraction between unlike components (CTAB and Tween-20) in the mixed micelles, ‘β’ value close to zero indicates nearly ideal mixing.33-35
Activity coefficients of surfactants in mixed micelles
Activity coefficients of surfactants in the mixed micelles are given by the relations:
γ1 = exp[β(1-X1)2] (6)
γ2 = exp[β(X1)2] (7)
where γ1 and γ2 are the activity coefficients of component 1 (CTAB), and component 2 (Tween-20), respectively, and the other parameters have their usual meanings described, above. Activity coefficients of CTAB and Tween-20 in the mixed micelle, thus obtained, are given in Table 4.
|
Mole fraction of CTAB in bulk (αCTAB) |
Activity coefficients of CTAB (γ1) in mixed micelle |
Activity coefficientsTween-20 (γ2) in mixed micelle |
|
0.4 |
0.460 |
0.934 |
|
0.5 |
0.554 |
0.922 |
|
0.6 |
0.512 |
0.852 |
|
0.8 |
0.175 |
0.834 |
Table 4 Activity coefficients γ1 and γ2 of CTAB and Tween-20, respectively, in their mixed micelles at 298.15K at different mole fractions of CTAB
The observed activity coefficients, less than unity, for the constituent surfactants indicate their low interaction in mixed micelles. Further, the observed lower activity coefficients of cationic surfactant CTAB than the non-ionic surfactant Tween-20 indicates that the former surfactant is deviated more from the standard than the later in the mixed micellar phase.36
Thermodynamic stability of mixed micelles
According to Sehgal et al.37 free energy of micellization (∆Gmic) of mixed surfactant system, based on the phase separation model, can be given by the relation:
∆Gmic = RT (Bo + B1X1 +B2X12) (8)
Where, R = Gas constant (8.314 JK-1mole-1), T = Temperature in Kelvin and X1 is the mole fraction of component 1(CTAB) in the micelle. The parameters B0, B1 and B2 are related through the following relations:
B0 = ln C2 (9)
Where C2 is the CMC of pure surfactant 2 (Tween-20).
ln(C2/C1) = B1 + B2 (10)
B2 = -β (11)
Where β, is the interaction parameter described in Section 3.4. The values of parameters B0, B1, B2 and free energy of micellization (∆Gmic) thus calculated for CTAB+Tween-20 mixed surfactant system at 298.15K and different mole fractions of CTAB in the bulk are presented in table 5. The calculated free energy of micellization of studied system are negative over the studied CTAB mole fractions. It suggests that the mixed micelles are more stable than the micelles of pure surfactants. A decrease of ∆Gmic with increasing mole fraction of CTAB in bulk suggests more stability of mixed micelles. The observed lowering of mixed CMC on mixing nonionic surfactant (Tween-20) indicates more stability of mixed micelle at higher concentrations of CTAB. This observed synergistic effect of mixed surfactant system in lowering and stabilizing the CMC may be exploited in pharmaceuticals6 for designing more efficient targeted drug delivery system, an improved performance in laundry for cleaning purpose, and in concentrating ores in the mineral floatation process,17 more efficient petroleum recovery in the tertiary process,16 and effectively managing the environmental pollution problem often caused due to coastal oil spills.
|
αCTAB |
B0 |
B1 |
B2 |
∆Gmic /KJMole-1 |
|
0.4 |
-7.01 |
-2.794 |
1.29 |
-18.79 |
|
0.5 |
-7.01 |
-2.610 |
1.11 |
-18.92 |
|
0.6 |
-7.01 |
-2.984 |
1.48 |
-19.40 |
|
0.8 |
-7.01 |
-2.303 |
0.78 |
-19.55 |
Table 5 Values of parameters B0, B1, B2 and free energy of micellization (∆Gmic) of CTAB+Tween-20 mixed surfactant system at 298.15K at different mole fractions of CTAB in the bulk (αCTAB)
Mixed micellar CMCs of a cationic surfactant cetyltrimethyl ammonium bromide (CTAB) and a non-ionic surfactant (Tween-20) have been determined at their varying bulk compositions. Using Rubingh’s regular solution theory for non-ideal mixing of surfactants systems, composition of mixed micelles, interaction parameters, activity coefficients of surfactants and Gibbs free energy of micellization have been determined. The results show that the observed synergism in mixed surfactants in lowering the mixed micelle CMC as well as enhancing its stability would be beneficial in pharmaceuticals for designing more efficient targeted drug delivery system, improved performance in laundry for cleaning purpose, more efficient petroleum recovery in the tertiary process, and effectively managing the environmental pollution problem due to coastal oil spills.
None.
Authors declare there are no conflicts of interests.

©2023 Merera, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.