Journal of
eISSN: 2473-0831


Research Article Volume 11 Issue 3
1Department of Biochemistry, University of Port Harcourt, Rivers State, Nigeria
2Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria
Correspondence: Kelechi N. Obi, Department of Biochemistry, University of Port Harcourt, Rivers State, Nigeria
Received: June 13, 2021 | Published: June 27, 2022
Citation: Obi KN, Uzoegwu PN. Methanol Root Extract of Clerodendrum capitatum Protected Wistar Albino Rats from Butylated Hydroxyl Toluene-Induced Oxidative Stress. J Anal Pharm Res. 2022;11(3):74-77 DOI: 10.15406/japlr.2022.11.00404
Clerodendrum capitatum has a wide range of ethnobiological applications which include, among others, treatment of erectile dysfunction, management of diabetes mellitus and cardiovascular diseases. The present study aimed to investigate the protective effects of methanol root extract of Clerodendrum capitatum (MECC) on Butylated hydroxyl Toluene (BHT) –induced oxidative stress in rats. The results show that MECC had protective effects as evidenced by normalisation of the activities of alkaline phosphatase (ALP), aspartate transaminase (AST), alanine transaminase (ALT) and concentrations of creatinine and urea which were all significantly (p<0.05) raised by BHT administration. Elevated bilirubin (total and direct) concentration was also reduced. The extract also raised the activities of superoxide dismutase (SOD), glutathione-S-transferase (GST) and catalase (CAT) which were all significantly reduced by BHT. Results also show that MECC pre-administration reduced the significantly elevated low density lipoprotein (LDL) and triglyceride (TG) concentrations, but had no significant effects on high density lipoprotein (HDL) and cholesterol levels. It may be concluded from this study that MECC had hepato- and nephro-protective potentials probably due to its antioxidant effects.
Key words: Clerodendrum capitatum, BHT, oxidative stress
Butylated hydroxyl toluene, BHT, is widely used as antioxidants for the preservation of food colour, flavor and nutritive value.1 Numerous studies have described the adverse effects of BHT which include pneumotoxicity2, hepatotoxicity3 and nephrotoxicity.4 The adverse effects of BHT is mainly due to metabolic activation to a highly electrophilic BHT quinone methide (BHT-QM) intermediate by cytochrome p450 monooxygenase.5 BHT-QM can form covalent adduct with macromolecules leading to the toxicities. In vivo, host defense mobilized against BHT-QM involve conjugation with glutathione catalyzed by glutathione-S-transferase (GST) system, but at high concentration, glutathione pool becomes depleted rendering tissue nucleophiles susceptible to adduct formation with electrophilic BHT-QM; hence, precipitating organ damage Since the utility of BHT and other industrial antioxidants is still under regulatory scrutiny, attention is being shifted to natural products as sources of more effective and safer antioxidants to improve the shelf-life of industrial products.
The genus Clerodendrum (Verbenacae) is very widely distributed in tropical and sub-tropical region of the world and is comprised of small trees, shrubs and herbs. The genus is taxonomically characterized by its toothed, oppositely arranged leaves, terminally or auxiliary cymose inflorescence, hypogenous bisexual flowers and persistence calyx. The genus exhibited a wide range of folk and indigenous medicinal uses. The roots of the plants are used traditionally in the management of erectile dysfunction in male.6 In Nigeria, it can be used traditionally for bone healing, management of diabetes obesity and hypertension. Other reported pharmacological properties include hypolipidemic,7 hepatoprotective activity against CCl4 induced liver injury in rats8 as well as serotorgenic activity.9
In this study, we investigated the protective effects of methanolic root extract of Clerodendrum capitatum (MECC) on butylated hydroxytoluene induced damage in rats having established that MECC has potent in vitro free radical scavenging activities and high phenolic contents.
Chemicals
Phosphate buffered saline, hydrogen peroxide, thiobabituric acid, ethanol, trichloroacetate, epinephrine, glutathione, sucrose, 1-chloro-2,4-dinitrobenzene, chloroform, methanol, ascorbic acid, thiourea were obtained from sigma Aldrich. Sodium carbonate, sodium hydroxide, sodium acetate, potassium dihydrogen phosphate, potassium iodide were obtained from BDH. Kits for lipid profile, kidney and liver function test were obtained from Randox, UK.
Experimental animals
The animals used for this study were Wister albino rats of about 8-10 weeks old. Sixteen (16) rats were purchased from the Biological Sciences animal house of the University of Nigeria, Nsukka. The rats were randomly distributed into four cages (four rats in each cage). The animals were maintained on poultry feed (Grand Cereals and Oil Mills Ltd, Nigeria) and tap water ad libitum. After a week of adaptation, they were subjected to various treatments for BHT-induced studies.
Preparation of plant extract
The root of Clerodendrum capitatum was collected from Imo state, Nigeria and was identified at the Herbarium, Department of Botany, University of Nigeria, Nsukka. The plant part was washed, air dried and reduced to fine powder by milling. The resulting powder was extracted with absolute methanol, filtered and concentrated using rotary evaporator and stored at 4oC for subsequent studies.
Animals treatments
The experiment was carried out according to the method described by10 with minor modifications. In brief, the rats were randomly divided into four groups of four animals each, namely:
Group A: vehicle control
Group B: BHT treated
Group C: BHT + MECC (500mg/kg)
Group D: BHT + MECC (1000mg/kg)
Group C and D were intragastrically pre-administered different doses of MECC while groups A and B were given the vehicle for one week. At the end of 7th day, 1000 mg/kg b.w of BHT dissolved in olive oil was given to groups B, C, D by gavage, 6 hrs after the extracts and vehicle were administered. At the same time, rat in group A were administered with the same volume of olive oil. All the rats were anaesthetized and sacrificed 18 hours later. Blood samples were collected by heart puncture while the livers were collected and homogenized for biochemical assays.
Serum analyses
The blood samples collected by heart puncture were centrifuged at 5000 rpm for 15 min, to obtain sera for biochemical analyses. Aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), blood urea nitrogen, creatinine, cholesterol, low density lipoproteins (LDL), very low density lipoprotein (VLDL), high density lipoprotein (HDL), total bilirubin (TBil) and direct bilirubin (DBil) were all determined according to the manufacturers instructions enclosed in Randox kits.
Tissue homogenate analyses
Liver homogenate was prepared (10% in phosphate buffer saline, pH 7.4) using a homogenizer. The homogenates were centrifuged at 9000 rpm for 30 mins and the supernatant obtained for various bioassays. Catalase (CAT) activity was determined according to Aebi.11 Superoxide dismutase (SOD) activity was determined by the method of Misra and Fridovich.12 Glutathione –S-transferase (GST) activity was determined by the method of Mozer.13 Protein concentration was determined by the method of Lowry.14
Statistical analyses
Statistical analyses were performed by oneway analysis of variance (ANOVA), followed by Least Significant Difference tests. A probability value of p<0.05 was considered significantly different. All experimental data were expressed as Mean ± SEM.
Effects of MECC on liver function in bht- induced oxidative stress
The effects of MECC on serum AST, ALP, ALT, Tbil and Dbil are presented in Table 1. Compared to the control group, and rats pre-administered MECC, results show that ALP and AST activities were significantly (p<0.05) increased in the BHT only rats. The serum activities of ALT, Dbil and Tbil levels also increased in the BHT only rats but not significantly compared to the control group (p>0.05). Pre-administration of MECC therefore significantly reduced the BHT-induced increase in the serum activities of heptic AST, ALT and ALP.
|
ALP (IU/L) |
ALT (IU/L) |
AST (IU/L) |
Tbil (mg/dl) |
Dbil (mg/dl) |
Control |
8.2 ± 5.32 |
27.66 ± 1.47 |
128.39 ± 12.89 |
7.59 ± 1.29 |
12.79 ± 0.46 |
BHT-only |
55.2 ± 5.88* |
34.60 ± 1.63 |
173.76 ± 4.47* |
11.35 ± 2.36 |
14.76 ± 1.00 |
BHT + 500 mg/kg b.w. |
35.16 ± 9.69 |
26.24 ± 2.93 |
137.66 ± 5.69 |
9.38 ± 2.01 |
10.50 ± 0.56 |
BHT + 1000 mg/kg b.w. |
20.85 ± 6.62 |
28.10 ± 1.17 |
107.40 ± 17.13 |
8.92 ± 2.42 |
11.92 ± 0.07 |
Table 1 Effect of MECC on alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), total bilirubin and direct bilirubin
Values are means ± SEM (n=4)
(*) significantly different (p<0.05) from the control group
Effects of MECC on Kidney Function in BHT-induced Oxidative Stress
As shown in Table 2, BHT significantly (p<0.05) raised the concentrations of urea and creatinine compared to the control group. Pre-administration of MECC at both 500 mg/kg b.w and 1000 mg/kg led to reduction of urea and creatinine to the level not significantly different (p<0.05) from normal group.
Urea (mg/dl) |
Creatinine (µmol/l) |
|
Control |
9.26 ± 0.344 |
0.32 ± 0.09 |
BHT-only |
13.04 ± 0.37* |
0.59 ± 0.03* |
BHT+500mg/kgbw |
6.26 ± 0.78 |
0.29 ± 0.09 |
BHT+1000mg/kgbw |
8.89 ± 0.50 |
0.25 ± 0.02 |
Table 2 Effects of MECC on Urea and Creatinine
Values are means ± SEM (n=4)
(*) significantly different (p<0.05) from the control group
Effects of Extract on Antioxidant Enzymes in BHT-induced Oxidative Stress
As shown in Figure 1A-C, BHT administration significantly (p<0.05) decreased the level of the enzymes SOD, GST and catalase compared to the control. Pre-treatment with MECC at both 500 mg/kg and 1000 mg/kg significantly enhanced the activities of these enzymes to the level not significantly (p>0.05) different from normal group.
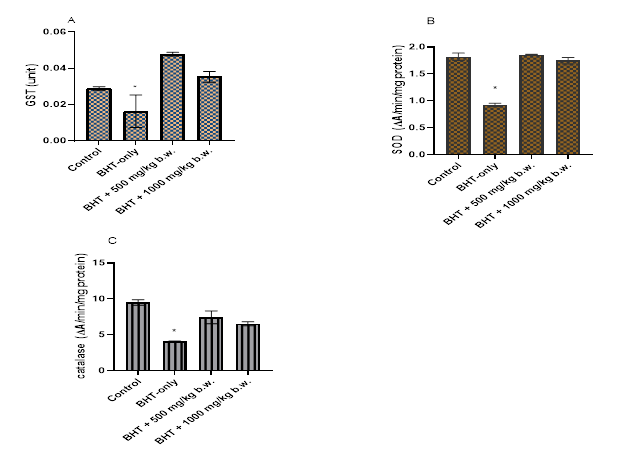
Figure 1 Effects of MECC on antioxidant Enzymes in the liver.
Data represent means ± SEM (n=4)
(*) significantly different (p<0.05) from the control group
Effects of Extract on Lipid Profile in BHT-induced Oxidative Stress
The result showed that administration of BHT increased serum triglyceride compared to the control group, though not significantly (p>0.05). the concentration of LDL was significantly increased. MECC pre-administration had no significant (p>0.05) effects, cholesterol, VLDL, and HDL concentration.
Butylated hydroxyl toluene has been used since 1950s for preservation of wide variety of consumer products, including foods, drugs and cosmetics. At high dose, BHT could induce hepatotoxicity10 and pneumotoxicit.2 In this study, the hepatic damage induced by BHT was evidenced by pathologic surge in alanine transaminase, aspartate transaminase, alkaline phosphatase and bilirubin (Table 1). Increases in the concentrations of urea and creatinine were also observed (Table 2) indicating that BHT at high concentration was nephrotoxic. The observations are in consistent with previous studies.10,15 Pre-administration of methanolic root extracts of Clerodendrum capitatum offered protective effects to liver and kidney as evidenced by reduction of transaminase activities, creatinine and urea concentrations. The toxic effects of BHT is due to its metabolic conversion to BHT quinone methide (BHT-QM) by CYP450 monooxygenases.
Antioxidant enzymes constitute the first line of defense against xenobiotics. Glutathioe-s-transferase is particularly important in the conjugation and hence inactivation of BHT-QM. In this study administration of BHT significantly reduced the activities of GST, SOD.and catalase (Figure1A-C). Results also showed that pre-administration of methanol root extract of Clerodendrun capitatum enhanced the activities of those enzymes. The induction of GST activity by the extracts is particularly important because of its direct involvement in detoxification of BHT-QM by conjugation with BHT.4 Plant phenolics are known to induce xenobiotic conjugating enzymes16–18 leading to modification of toxic effects of xenobiotics such as BHT. Previous studies have indicated there is no significant lipid peroxidation in BHT intoxication10,19 Thus, the toxic effect of BHT is probably not due to lipid peroxidation but by other mechanisms including glutathione (GSH) depletion.10
Dislipidemia is a vital component of cardiovascular diseases. Elevated low-density lipoproteins (LDL), very low-density lipoproteins (VLDL), cholesterol, and reduced concentration of high density lipoproteins (HDL) are all cardiovascular risk factors.20 In this study, it was observed that BHT altered significantly elevated the concentration of LDL, the bad cholesterol (Table 3). The study also showed that pre- administration of MECC significantly reduced the concentrations of LDL. HDL and VLDL were however, not significantly affected by both BHT and the extract. This finding corroborates19 where prevention of pathologic alteration in lipid profile induced by BHT was demonstrated. The reduction of LDL concentration is clinically beneficial due to the implication of LDL in the pathogenesis of cardiovascular diseases.21 The ability of the plant extract to inhibit oxidation of LDL has equally been reported22,23, and may account for inverse association between dietary flavonoid intake and decrease in mortality due to heart diseases.23-25 Thus, MECC may find use in prevention, management or treatment of cardiovascular diseases which normally implicate altered lipid profile.
|
Cholesterol |
TG |
LDL |
VLDL (mmol/L) |
Control |
1.06 ± 0.56 |
1.49 ± 0.39 |
1.34 ± 0.07 |
0.30 ± 0.02 |
BHT-only |
1.03 ± 0.18 |
1.89 ± 0.37 |
2.75 ± 0.22* |
0.36 ± 0.08 |
BHT + 500 mg/kg b.w. |
1.12 ± 0.27 |
1.41 ± 0.02 |
1.65 ± 0.31 |
0.35 ± 0.04 |
BHT + 1000 mg/kg b.w. |
0.99 ± 0.04 |
1.82 ± 0.09 |
1.56 ± 0.25 |
0.45 ± 0.10 |
Table 3 Effects of MECC on lipid profile
Values are Mean ± SEM (n=4)
(*) significantly different (p<0.05) from the control group
The results of this study demonstrate that MECC could prevent acute BHT- induced oxidative organ damage in Wistar rats as evidenced by the reduction of elevated disease markers, and enhancement of antioxidant enzymes in the liver.
The authors wish to express deep appreciation to Engr. Uche Obi for his invaluable contributions to the success of this research.
The authors declare that there is no conflict of interest.
None.

©2022 Obi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.