Journal of
eISSN: 2473-0831


Review Article Volume 2 Issue 5
Correspondence: Saleh S Altayyar, Biomedical Technology Department, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia, Tel 505456035
Received: June 07, 2016 | Published: July 5, 2016
Citation: Altayyar SS (2016) Medical Devices and Patient Safety. J Anal Pharm Res 2(5): 00034. DOI: 10.15406/japlr.2016.02.00034
There is a worldwide requirement today for accreditation and quality standards in Healthcare. In all the standards and regulatory bodies, there are strong components of medical devices management, such as medical device defect reporting, incident management and reporting, and risk management. Medical devices represents the most sizable investment in hospitals, they play an important role in the diagnosis, Prevention, monitoring, treatment, or alleviation of disease. Medical devices are regulated throughout their life span from conception and development to disposal. The objective of medical devices regulations is ensuring their safety, quality, effectiveness, performance according to the intended purpose. There is significant risk to staff and patients from use and/or misuse of medical devices. However, appropriate implementation of medical devices regulation will enhance public health, patient safety, and the safety of health care professionals and environment. Only 65% of 145 countries have national authorities responsible for implementing and enforcing medical devices regulations. This paper will look at the three stages of medical devices regulation (i.e. pre market, on market, and post market), and will primarily focus on the post market surveillance as a monitoring activities of medical devices in use, such as collection of information on the quality, safety and performance of medical devices. It will also shade the light on the medical devices hazards and adverse events reporting, the type of reported adverse events, and their source.
Keywords: Medical device, Post market surveillance, Adverse events, Recall
Medical devices represent the most sizable investment in Hospitals. In general, there are wide variations in practices between different health systems and hospitals within the same system in the range, type and costs for medical equipment management. There is significant risk to staff and patients from use and/or misuse of medical equipment. Medical devices range from items as simple as tongue depressors to more complex and high risk devices, such as stents, base makers, and ventilators. Almost all regulatory authorities and the WHO agree on one basic definition for medical device which is “an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including a component part, or accessory which is:
Regulatory system for medical devices are less developed than those for other health products such as medicines and vaccines, the formal regulation of medical devices began in the mid-1990.3 65 % of 145 countries have a national authorities responsible for implementing and enforcing medical devices regulations, and many of those governments that have drafted regulations have made little progress in implementing them.4 The main objective of Medical Devices Regulations is to ensure their Safety, Quality. Effectiveness and Performance according to the intended purpose. Therefore, a medical device is classified according to the risk it poses to the patient and/or to the user. The primary purpose of the regulatory classification system is to establish the level of regulatory control required for the marketing of the device. Figure 1 illustrates the level of regulatory requirements for each class of medical device.5
A medical device should be regulated throughout its life cycle i.e. conception and development to retirement and disposal (Figure 2).6 User familiarity with the indications, contra-indications and operating procedures recommended by the manufacturer, and user’s qualifications and training in the proper use of the device are very crucial for the device safety in actual use.
Post market surveillance
Post market surveillance is a broad term that covers all monitoring activities of medical devices. The Global Harmonization Task Force (GHTF) member economies (Australia, Canada, European Union, and USA) requirements for the post market surveillance/vigilance system cover after sale obligations, monitoring device performance, problem identification, adverse events reporting, alert, recall, and corrective action.7 Post market surveillance can be divided in proactive and reactive activities, where proactive activities pertaining to market control such as batch release testing and establishment’s inspection, and reactive activities cover the medical device vigilance system. The post market surveillance information are useful for Injury prevention, product improvement, development of standards, and regulatory refinement. The medical industry divides the medical electronics into three categories, diagnostic and therapy equipment, home equipment and imaging equipment (Figure 3).8
Medical Device Hazards
Hazard is a potential source of harm, and it is due to inherent risk of medical treatment, device failure, device malfunction, and device use (Figure 4),9 Use – Related Hazards are Hazards caused by how a device is used. It has been suggested that the frequency and consequence of hazards resulting from medical device use might far exceeds those resulting from device failure.10,11 It has been reported that 98000 people die in hospitals in any given year as a result of medical errors.12
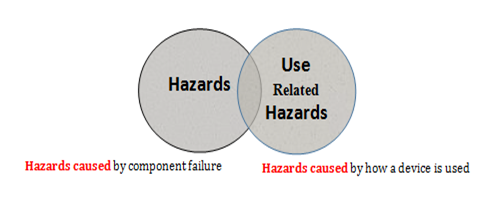
Figure 4 Device Failure Hazards and use Related Hazards.
Hazards caused by how a device is used Hazards caused by component failure
Use related hazards can occur for many reasons, among which are using the device in ways that were not anticipated, device use is inconsistent with the user’s expectations about the device operation, and the use environment such as work load and mental load9. Alarm hazards. Inadequate reprocessing of medical devices, and patient handling of the device are examples of device failure hazards.13
Adverse events and recalls
Adverse events of medical devices such as ‘arching’ and ‘burns’ from defibrillator pads must be reported to the regulatory authority in the country where the incident happens, unreported problems increase the potential for harm to patients and healthcare professionals. Reporting adverse events can help regulatory authorities taken preventive measure, investigate, work with manufacturers on corrective actions, and disseminate reports to other regulatory authorities and reporting centers such as the National Competent Authority Report (NCAR) of International Medical Device Regulators Forum (MDIRF), and the Asian Harmonization Working Party (AHWP) Safety Alerts Dissemination System (SADS).The number of adverse events submitted to the USFDA by manufacturers, user facilities, and other reporters doubled from 2003 to 2007, the overall medical device adverse event reports submitted in 2003 were 72,866 , increasing to 150,210 reports in 2007.14 The majority of these reports were submitted by manufacturers. The medical device recalls by manufacturers is very important as it could be used to take preventive measures to prevent the occurrence of incidents that may leads to injuries and deaths. The total number of recalls reported by the USFDA for the period 1999 – 2005 was 3771.15 The Therapeutic Good Administration (TGA) received a total of 32,300 medical device adverse events reports during the period 1986 to 2013, with 3309 of those received in 2013,16 and the majority of these reports were made by sponsors of medical devices. The Saudi FDA reported 705 adverse events during the period (2008 to 2015), the majority of these reports (83%).were reported by manufactures and vendors, and 12% were reported by healthcare providers. 7.5% of these reports were death, 9.2% were injuries, and 42% were malfunction. The total number of Adverse Events reported by the Saudi FDA during the period (2008 - 2015) by type of outcome of the adverse event is shown in Table 1 and Figure 5 and Figure 6.17
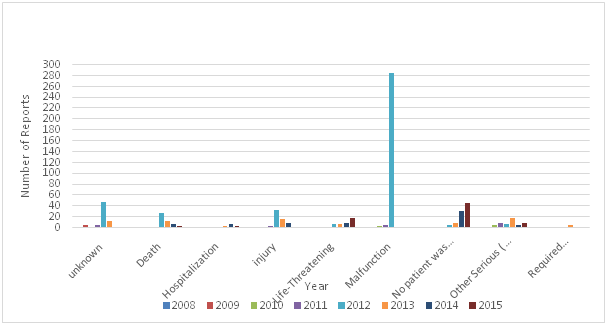
Figure 5 The total number of adverse events reported by Saudi FDA during the period (2008-2015) by type of outcome of the adverse event.
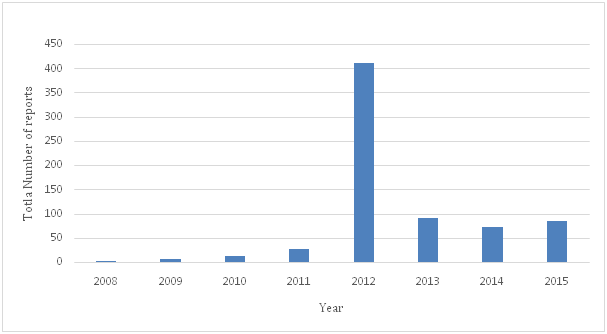
Figure 6 The total number of adverse events reported by the Saudi FDA during the period (2008-2015) by all types of outcome of adverse events.
|
Year |
Unknown |
Death |
Hospitalization |
Injury |
Life-threatening |
Malfunction |
No patient was affected |
Other serious (important medical events) |
Required intervention |
Total |
|
2008 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
|
2009 |
5 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
0 |
7 |
|
2010 |
2 |
0 |
0 |
0 |
0 |
4 |
0 |
6 |
0 |
12 |
|
2011 |
5 |
2 |
0 |
3 |
2 |
5 |
0 |
9 |
0 |
26 |
|
2012 |
48 |
27 |
0 |
33 |
6 |
285 |
4 |
7 |
1 |
411 |
|
2013 |
13 |
13 |
4 |
17 |
8 |
2 |
9 |
19 |
5 |
90 |
|
2014 |
0 |
8 |
7 |
9 |
10 |
0 |
32 |
5 |
2 |
73 |
|
2015 |
0 |
3 |
3 |
2 |
19 |
0 |
46 |
9 |
2 |
84 |
|
Total |
75 |
53 |
14 |
65 |
45 |
296 |
91 |
56 |
10 |
705 |
Table 1 Total number of Adverse Events reported by the Saudi FDA during the period (2008 - 2015) by type of outcome of the adverse event17
The total estimate of medical device associated adverse events from emergency departments of a national stratified probability sample of hospitals was 454385, which is over four times greater than the annual number of adverse event reports received by medical device regulatory surveillance systems,18 unintentional traumatic events associated with a particular device was reported as the most common mechanism of injury. This emphasizes those medical device-associated adverse events as an under-recognized public health problem.
According to the European Medical Devices Directive (93/42/EEC) medical device manufacturers are legally required to report adverse incidents and Field Safety Corrective Actions (FSCAs) to Competent Authorities. The European Commission’s Guidelines document on a medical devices vigilance system (MEDDEV 2.12 -1 rev 8 January 2013) state that, manufacturers are required to notify the relevant national competent authority about incidents and field safety corrective actions (section 5.1 and 5.4). Manufacturer is also required to investigate incidents and take necessary corrective action (section 5.2 and 5.3). Detailed information on timelines and other vigilance reporting requirements are available in European Commission’s Guidance document MEDDEV 2.12/1. There may be financial penalties or criminal sentences for companies that fail to correctly report incidents.
The European vigilance system requires user to report an incident to the manufacturer or competent authority if there is a clear link between the device and the incident, and / or if the incident has led or might have led to death, and / or deterioration in patient or user health the user, The manufacturer or the authorized representative are required to submit initial incident report to the competent authority followed by a final report.19
Under the European medical devices vigilance system, there are cases when reporting is not required, such as when deficiencies in the device is found prior to use, the incident is caused by patient conditions, or when the incident did not lead to death or serious deterioration in state of health.20
The Medical Product Safety objectives for 2020 focus on overall improvement of patient treatment and appropriate use of medical products such as medical devices. These objectives reflect strong scientific support for safe use of medical products, which promotes better health among Americans.21 Increasing appropriate use and monitoring adverse effects of medical products will decrease adverse events and harmful reactions by focusing on safety efforts, improve the overall effectiveness of treatment by reducing harm from medical product.21 The number and complexity of medical devices is growing rapidly, and they have become smaller and more portable. Patients and their family are becoming more knowledgeable about medical devices. Therefore, a great need for increase awareness of incidents that potentially involve a medical device or technology not only to healthcare practitioners, but also to patients and their families. Patients and users education on the risk associated with medical devices and appropriate use of medical devices will contribute highly to the reduction or elimination of medical devices incidents. It will increase adverse events reporting and enhance patient’s safety.
None.
The authors declare there is no conflict of interests.
None.

©2016 Altayyar. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.